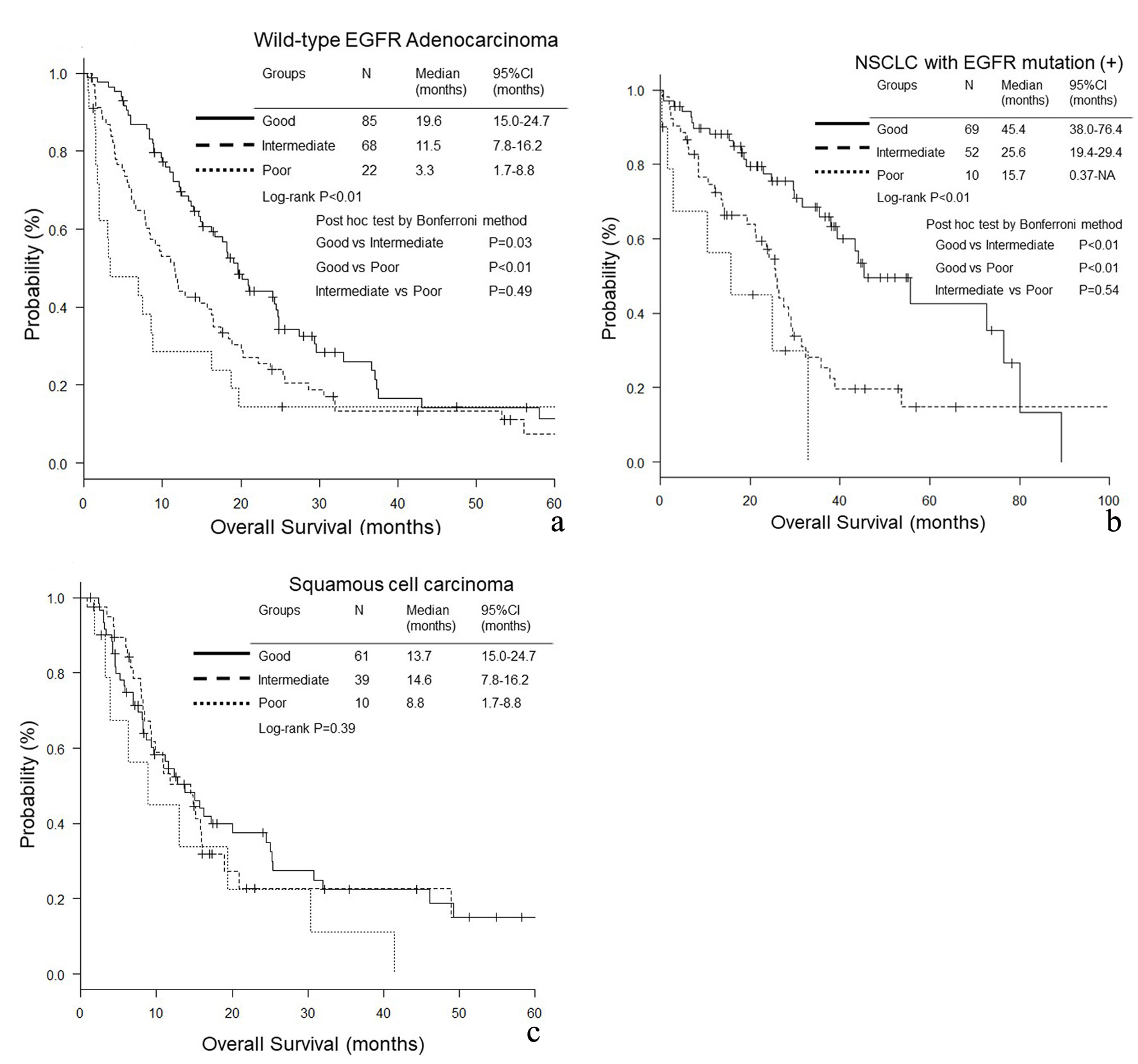
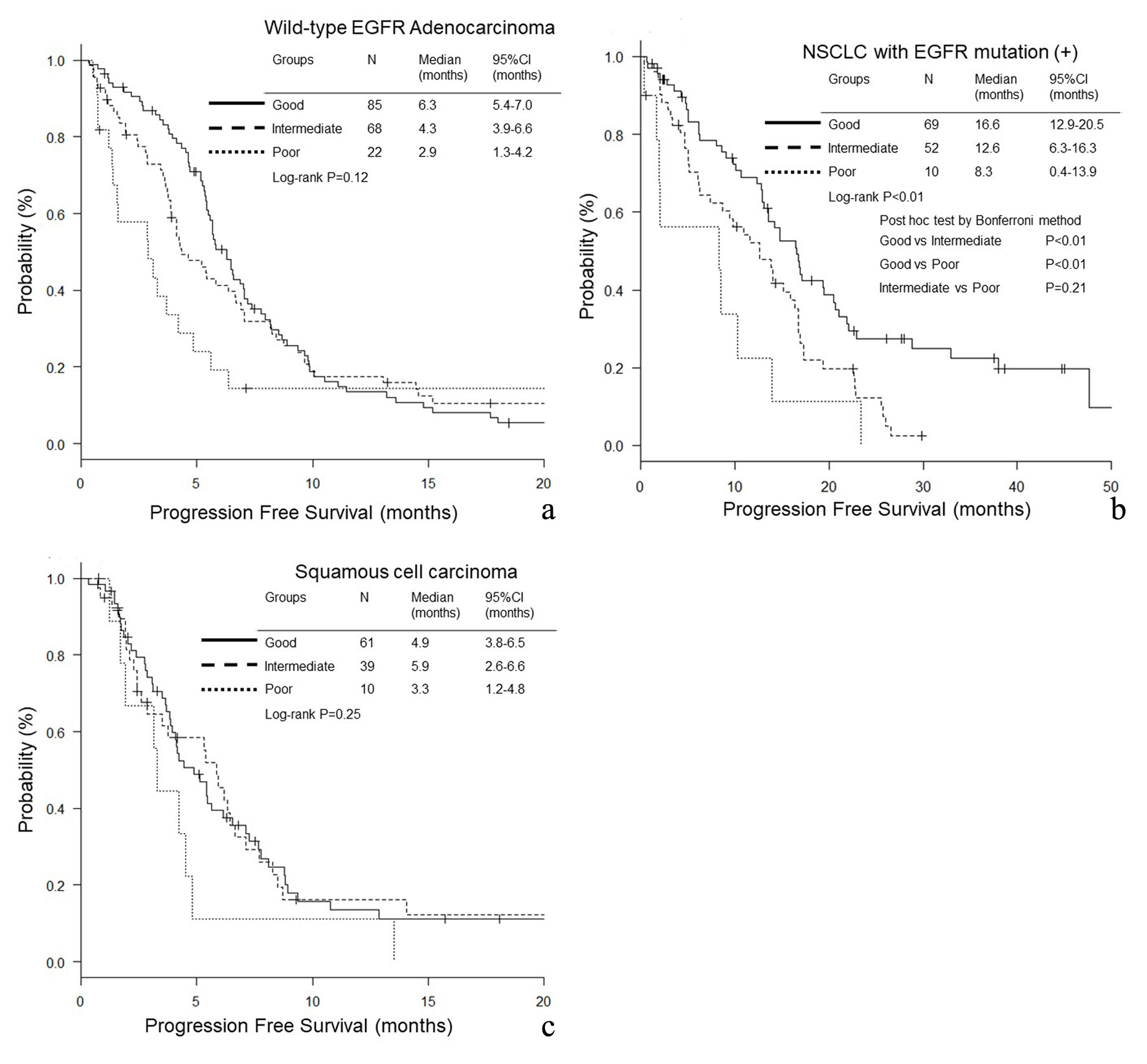
Figure 1. Overall survival (OS) according to lung immune prognostic index (LIPI) groups. (a) Adenocarcinoma without any driver mutations. (b) NSCLC with EGFR mutation. (c) Squamous cell carcinoma.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 10, Number 1, February 2019, pages 35-45
Pretreatment Lung Immune Prognostic Index Is a Prognostic Marker of Chemotherapy and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
Figures

Tables
| All | LIPI | P | |||
|---|---|---|---|---|---|
| Good | Intermediate | Poor | |||
| CI: confidence interval; DCR: disease control rate; dNLR: derived neutrophil-to-lymphocyte ratio; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; IQR: interquartile range; LDH: lactate dehydrogenase; LIPI: lung immune prognostic index; NA: not assessed; ORR: overall response rate; ULN: upper limit of normal. aFisher’s exact test; bKruskal-Wallis rank sum test. | |||||
| N | 175 | 85 | 68 | 22 | |
| Backgrounds | |||||
| Sex (N), male/female | 120/55 | 55/30 | 47/21 | 18/4 | 0.33a |
| Age (years), median (IQR) | 68 (62.4 - 74.6) | 68 (63.5 - 74.9) | 65 (60.2 - 74.5) | 69 (64.0 - 71.5) | 0.49b |
| Smoking status, none/former/current smokers | 30/56/89 | 14/26/45 | 13/24/31 | 3/6/13 | 0.85a |
| PD-L1 status, ≥ 50%/1-49%/< 1%/NA | 8/5/1/161 | 6/4/1/74 | 2/0/0/66 | 0/0/0/22 | 0.25a |
| ECOG-PS, 0 - 1/≥ 2 | 136/39 | 76/9 | 52/16 | 8/14 | < 0.01a |
| Stage, IIIB/IV/recurrence | 32/132/11 | 20/57/8 | 12/56/0 | 0/19/3 | < 0.01a |
| Metastatic sites, ≥ 2 | 89 | 38 | 33 | 18 | 0.06a |
| First-line chemotherapy | |||||
| Regimen | |||||
| Single/combination (N) | 7/168 | 4/81 | 3/65 | 0/22 | 0.88a |
| Pemetrexed-containing (N) | 84 | 40 | 33 | 11 | 0.96a |
| Bevacizumab-containing | 35 | 24 | 9 | 2 | 0.03a |
| Efficacy | |||||
| ORR (%) (95% CI) | 39.4 (32.1 - 47.1) | 55.3 (44.1 - 66.1) | 30.9 (20.2 - 43.3) | 18.2 (5.2 - 40.3) | < 0.01a |
| DCR (%) (95% CI) | 70.9 (63.5 - 77.5) | 82.4 (72.6 - 89.8) | 64.7 (52.2 - 75.9) | 45.5 (24.4 - 67.8) | < 0.01a |
| Second and further line | |||||
| Second-line (N) | 107 | 57 | 41 | 9 | 0.08a |
| Immuno-checkpoint inhibitor (N) | 25 | 18 | 7 | 0 | 0.02a |
| Laboratory data | |||||
| dNLR | |||||
| Median (IQR) | 2.08 (1.61 - 2.98) | 1.82 (1.30 - 2.06) | 2.69 (1.89 - 3.12) | 3.96 (3.64 - 5.58) | < 0.01b |
| ≥ 3 (N) | 132 | 85 | 47 | 0 | < 0.01a |
| LDH | |||||
| Median (IQR) | 203 (170 - 265) | 174 (154 - 200) | 247 (207 - 311) | 298 (244 - 415) | < 0.01b |
| > ULN (N) | 69 | 0 | 47 | 22 | < 0.01a |
| All | LIPI | P | |||
|---|---|---|---|---|---|
| Good | Intermediate | Poor | |||
| CI: confidence interval; DCR: disease control rate; dNLR: derived neutrophil-to-lymphocyte ratio; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; EGFR: epidermal growth factor receptor; IQR: interquartile range; LDH: lactate dehydrogenase; LIPI: lung immune prognostic index; NA: not assessed; ORR: overall response rate; TKI: tyrosine kinase inhibitor; ULN: upper limit of normal. aFisher’s exact test; bKruskal-Wallis rank sum test. | |||||
| N | 131 | 69 | 52 | 10 | |
| Backgrounds | |||||
| Sex (N), male/female | 52/79 | 28/41 | 20/32 | 4/6 | 0.96a |
| Age (years), median (IQR) | 73 (65 - 78) | 74 (67 - 78) | 70.5 (63.8 - 77) | 65 (50 - 81.8) | 0.29b |
| Smoking status, non/former/current smokers | 63/41/27 | 37/21/11 | 22/17/13 | 4/3/3 | 0.31a |
| EGFR mutation status, Ex19el/Ex21 point/minor | 129/56/7 | 34/31/4 | 27/22/3 | 7/3/0 | 0.87a |
| PD-L1 status, ≥ 50%/1-49%/< 1%/NA | 3/4/5/119 | 3/1/4/61 | 0/3/1/48 | 0/0/0/10 | 0.49a |
| ECOG-PS, 0 - 1/≥ 2 | 93/37 | 55/14 | 37/15 | 2/8 | < 0.01a |
| Stage, III/IV/recurrence | 10/90/31 | 7/42/20 | 2/39/11 | 1/9/0 | 0.12a |
| Metastatic sites, ≥ 2 | 91 | 43 | 41 | 7 | 0.15a |
| EGFR-TKI | |||||
| Regimen, gefitinib/erlotinib/afatinib | 80/38/13 | 43/21/5 | 33/13/6 | 4/4/2 | 0.42a |
| Line, first/second or further | 99/32 | 53/16 | 37/15 | 9/1 | 0.46a |
| Efficacy | |||||
| ORR (%) (95% CI) | 64.1 (55.3 - 72.3) | 68.1 (55.8 - 78.8) | 63.5 (49.0 - 76.4) | 40.0 (12.2 - 73.8) | 0.26a |
| DCR (%) (95% CI) | 82.4 (74.8 - 88.5) | 85.5 (75.0 - 92.8) | 82.7 (69.7 - 91.8) | 60.0 (26.2 - 87.8) | 0.16a |
| Post-EGFR-TKI therapy | |||||
| Further-line | 68 | 30 | 30 | 8 | 0.06a |
| Osimeritinib | 26 | 9 | 13 | 4 | 0.06a |
| Immuno-checkpoint inhibitor | 5 | 4 | 1 | 0 | 0.59a |
| Laboratory data | |||||
| dNLR | |||||
| Median (IQR) | 1.98 (1.44 - 2.72) | 1.67 (1.31 - 2.04) | 2.20 (1.57 - 3.10) | 3.43 (3.27 - 3.83) | < 0.01b |
| ≥ 3 (N) | 25 | 0 | 15 | 10 | < 0.01a |
| LDH | |||||
| Median (IQR) | 203 (176.5 - 240.5) | 183 (163 - 198) | 242 (211.8 - 308) | 247 (232.5 - 466.5) | < 0.01b |
| > ULN | 25 | 0 | 37 | 10 | < 0.01a |
| All | LIPI | P | |||
|---|---|---|---|---|---|
| Good | Intermediate | Poor | |||
| CI: confidence interval; DCR: disease control rate; dNLR: derived neutrophil-to-lymphocyte ratio; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; IQR: interquartile range; LDH: lactate dehydrogenase; LIPI: lung immune prognostic index; NA: not assessed; nab-PTX: nanoparticle albumin-bound paclitaxel; ORR: overall response rate; PTX: paclitaxel; ULN: upper limit of normal. aFisher’s exact test; bKruskal-Wallis rank sum test. | |||||
| N | 110 | 61 | 39 | 10 | |
| Backgrounds | |||||
| Sex (N), male/female | 85/25 | 49/12 | 28/11 | 8/2 | 0.59a |
| Age (years), median (IQR) | 71.5 (65 - 76) | 72 (65 - 76) | 70 (66 - 75) | 71.5 (62 - 75) | 0.62b |
| Smoking status, non/former /current smoker | 8/42/60 | 4/19/38 | 3/20/16 | 1/3/6 | 0.23a |
| PD-L1 status, ≥ 50%/1-49% /< 1%/NA | 1/1/2/106 | 1/0/1/59 | 0/1/1/37 | 0/0/0/22 | 1.00a |
| ECOG-PS, 0-1/≥ 2 | 83/27 | 50/11 | 28/11 | 5/5 | 0.08a |
| Stage, IIIB/IV/recurrence | 40/57/13 | 25/25/11 | 14/23/2 | 1/9/0 | 0.03a |
| Metastatic sites, ≥ 2 | 33 | 16 | 13 | 4 | 0.52a |
| First-line chemotherapy | |||||
| Regimen | |||||
| Single/combination (N) | 11/89 | 8/53 | 2/37 | 1/9 | 0.39a |
| PTX or nab-PTX | 72 | 36 | 28 | 8 | 0.29a |
| Efficacy | |||||
| ORR (%) (95% CI) | 42.7 (33.3 - 52.5) | 45.9 (33.1 - 59.2) | 35.9 (21.2 - 52.8) | 50.0 (18.7 - 81.3) | 0.60a |
| DCR (%) (95% CI) | 65.5 (55.8 - 74.3) | 63.9 (50.6 - 75.8) | 69.2 (52.4 - 83.0) | 60.0 (26.2 - 87.8) | 0.79a |
| Second and further line | |||||
| Second-line (N) | 66 | 39 | 23 | 4 | 0.34a |
| Immuno-checkpoint inhibitor (N) | 10 | 5 | 5 | 0 | 0.61a |
| Laboratory data | |||||
| dNLR | |||||
| Median (IQR) | 2.25 (1.73 - 3.13) | 2.00 (1.69 - 2.39) | 3.20 (1.88 - 4.04) | 3.39 (3.19 - 3.91) | < 0.01b |
| ≥ 3 (N) | 30 | 0 | 20 | 10 | < 0.01a |
| LDH | |||||
| Median (IQR) | 198.5 (168 - 229) | 181 (158 - 202) | 224 (189.5 - 277.5) | 282 (243 - 304.5) | < 0.01b |
| > ULN | 29 | 0 | 19 | 10 | < 0.01a |
| Ad, EGFR mt (-) | NSCLC, EGFR mt (+) | SQ | |
|---|---|---|---|
| N | 175 | 131 | 110 |
| Survival | |||
| Dead | 137 | 71 | 79 |
| At our hospital | 108 | 38 | 61 |
| At other institutions | 15 | 23 | 11 |
| At home | 14 | 10 | 7 |
| Alive | 20 | 37 | 10 |
| Lost follow-up | 18 | 23 | 21 |
| Chemotherapy | |||
| Confirmed PD or death | 154 | 103 | 88 |
| Continued | 2 | 17 | 0 |
| Discontinued | 173 | 86 | 110 |
| PD | 89 | 73 | 36 |
| Completion of pre-defined courses | 21 | 0 | 27 |
| Adverse effects | 29 | 13 | 18 |
| Deteriorated conditions | 25 | 12 | 24 |
| Refusal | 8 | 4 | 3 |
| Transfer to other hospitals | 1 | 5 | 0 |
| Unknown cause | 0 | 3 | 2 |
| Variable | Ad, EGFR mt (-), HR (95% CI) | P | NSCLC, EGFR mt (+), HR (95% CI) | P | SQ, HR (95% CI) | P |
|---|---|---|---|---|---|---|
| Ad: adenocarcinoma, CI: confidence interval, ECOG-PS: Eastern Cooperative Oncology Group Performance Status; EGFR: epidermal growth factor receptor; HR: hazard ratio; LIPI: lung immune prognostic index; nab-PTX: nanoparticle albumin-bound paclitaxel; NSCLC: non-small cell carcinoma; SQ: squamous cell carcinoma; TKI: tyrosine kinase inhibitor. | ||||||
| Age, years | ||||||
| < 70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 70 | 1.29 (0.90 - 1.85) | 0.16 | 1.95 (1.13 - 3.35) | 0.02 | 0.90 (0.56 - 1.45) | 0.66 |
| Smoking history | ||||||
| Non-smoker | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Smoker | 1.41 (0.88 - 2.28) | 0.16 | 1.07 (0.64 - 1.79) | 0.81 | 1.87 (0.57 - 6.20) | 0.30 |
| No. of metastatic sites | ||||||
| < 2 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 2 | 0.88 (0.62 - 1.26) | 0.49 | 2.30 (1.26 - 4.22) | < 0.01 | 1.51 (0.90 - 2.54) | 0.12 |
| ECOG-PS | ||||||
| 0 or 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 2 | 3.12 (2.04 - 4.79) | < 0.01 | 2.54 (1.33 - 4.86) | < 0.01 | 3.14 (1.87 - 5.30) | < 0.01 |
| Line of EGFR-TKI | ||||||
| First-line | 1 (Reference) | |||||
| Second or later line | 0.71 (0.40 - 1.25) | 0.24 | ||||
| Albumin level (g/dL) | ||||||
| ≥ 3.5 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| < 3.5 | 1.56 (1.05 - 2.32) | 0.03 | 0.59 (0.31 - 1.10) | 0.10 | 1.17 (0.71 - 1.93) | 0.54 |
| LIPI | ||||||
| Good | 1(Reference) | 1 (Reference) | 1 (Reference) | |||
| Intermediate | 1.49 (1.03 - 2.15) | 0.04 | 2.30 (1.33 - 3.99) | < 0.01 | 1.01 (0.61 - 1.67) | 0.98 |
| Poor | 1.67 (0.94 - 2.98) | 0.08 | 2.76 (1.03 - 7.42) | 0.04 | 1.64 (0.78 - 3.44) | 0.19 |
| Variable | Ad, EGFR mt (-) | P | NSCLC, EGFR mt (+) | P | SQ | P |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Ad: adenocarcinoma; CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; EGFR-TKI: epidermal growth factor receptor-tyrosine kinase inhibitor; HR: hazard ratio; LIPI: lung immune prognostic index; nab-PTX: nanoparticle albumin-bound paclitaxel; NSCLC: non-small cell carcinoma; PTX: paclitaxel; SQ: squamous cell carcinoma. | ||||||
| Age, years | ||||||
| < 70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 70 | 1.09 (0.77 - 1.53) | 0.62 | 1.25 (0.82 - 1.90) | 0.30 | 1.13 (0.71 - 1.80) | 0.61 |
| Smoking history | ||||||
| Non-smoker | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Smoker | 1.01 (0.65 - 1.58) | 0.96 | 1.08 (0.72 - 1.60) | 0.72 | 1.22 (0.53 - 2.82) | 0.64 |
| No. of metastatic sites | ||||||
| < 2 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 2 | 0.99 (0.71 - 1.38) | 0.94 | 1.52 (0.94 - 2.45) | 0.09 | 1.10 (0.66 - 1.85) | 0.72 |
| ECOG-PS | ||||||
| 0 or 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 2 | 1.90 (1.26 - 2.86) | < 0.01 | 1.48 (0.86 - 2.55) | 0.16 | 1.82 (1.04 - 3.18) | 0.04 |
| Bevacizumab-containing | ||||||
| Yes | 1 (Reference) | |||||
| No | 1.40 (0.93 - 2.12) | 0.11 | ||||
| PTX or nab-PTX-containing | ||||||
| Yes | 1 (Reference) | |||||
| No | 1.53 (0.95 - 2.45) | 0.08 | ||||
| EGFR-TKI line | ||||||
| First | 1 (Reference) | |||||
| Second or later | 0.76 (0.47 - 1.21) | 0.24 | ||||
| Albumin level, g/dL | ||||||
| ≥ 3.5 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| < 3.5 | 1.15 (0.79 - 1.67) | 0.48 | 0.80 (0.46 - 1.37) | 0.41 | 0.90 (0.57 - 1.44) | 0.67 |
| LIPI | ||||||
| Good | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Intermediate | 1.00 (0.70 - 1.43) | 1.00 | 1.57 (1.01 - 2.44) | 0.04 | 1.08 (0.67 - 1.73) | 0.75 |
| Poor | 1.35 (0.78 - 2.34) | 0.29 | 2.63 (1.14 - 6.07) | 0.02 | 2.00 (0.94 - 4.22) | 0.07 |