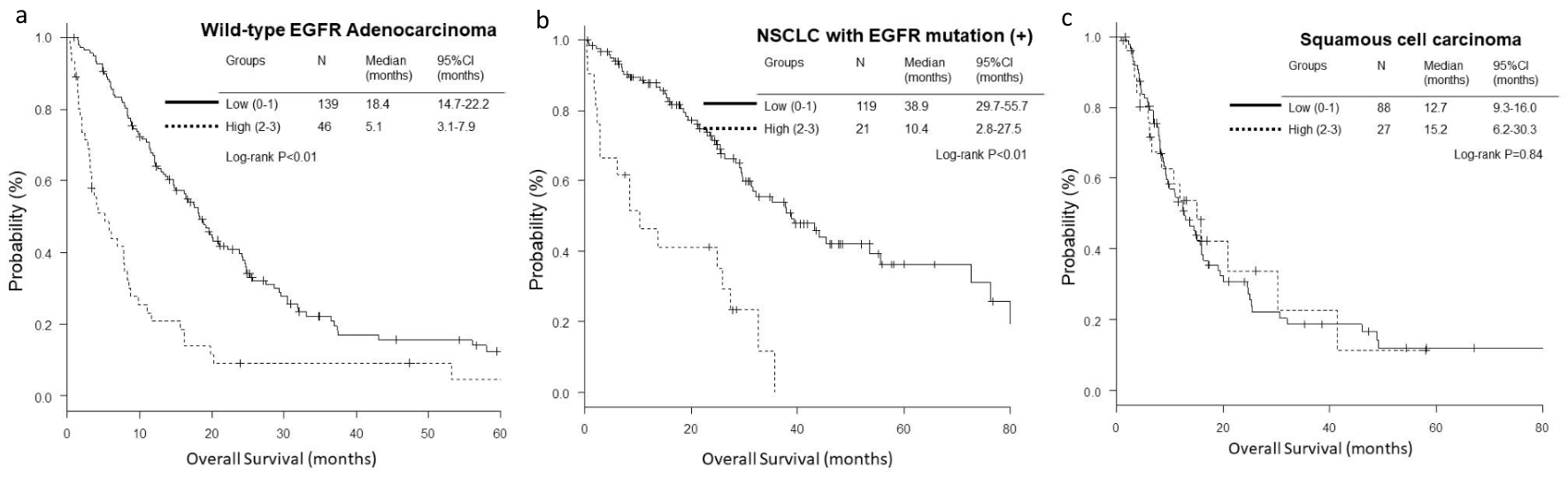
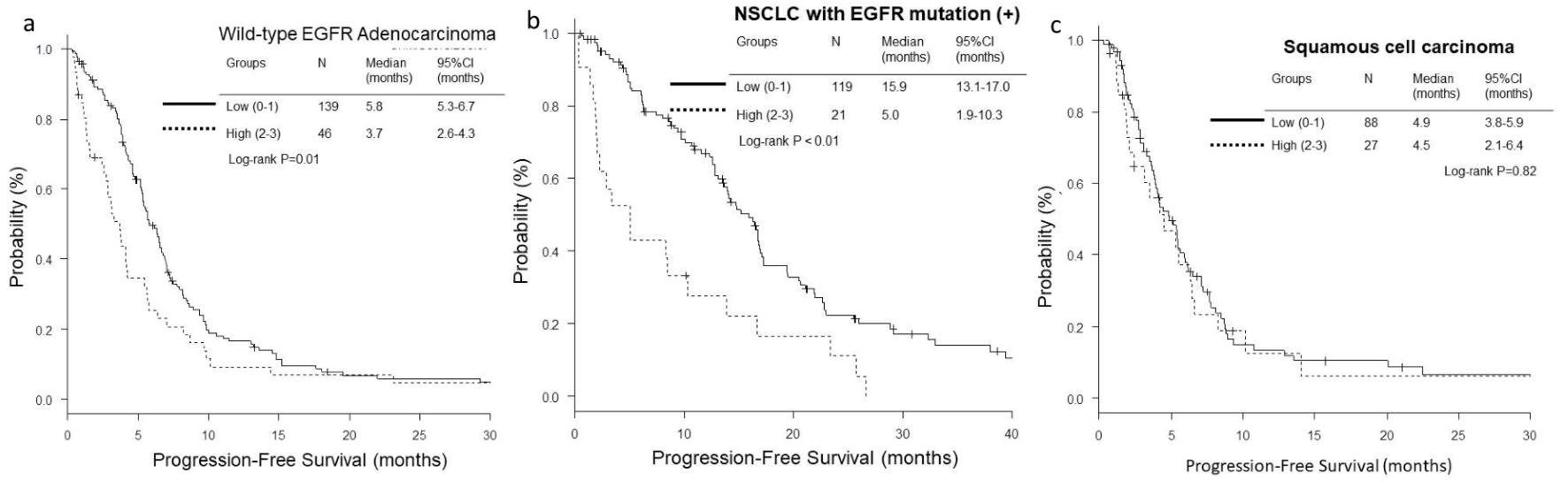
Figure 1. Kaplan-Meier curves of overall survival (OS) according to GRIm-Score. (a) Wild-type EGFR adenocarcinoma. (b) NSCLC with activated EGFR mutation. (c) Squamous cell carcinoma.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 10, Number 1, February 2019, pages 55-61
Gustave Roussy Immune Score Is a Prognostic Factor for Chemotherapy-Naive Pulmonary Adenocarcinoma With Wild-Type Epidermal Growth Factor Receptor
Figures

Tables
| All | Low | High | P | |
|---|---|---|---|---|
| CI: confidence interval; DCR: disease control rate; ECOG-PS: Eastern Cooperative Oncology Group performance status; IQR: interquartile range; LDH: lactate dehydrogenase; NA: not assessed; NLR: neutrophil-to-lymphocyte ratio; ORR: overall response rate; ULN: upper limit of normal. aFisher’s exact test; bMann-Whitney U test. | ||||
| N | 185 | 139 | 46 | |
| Backgrounds | ||||
| Sex (N), men/women | 128/57 | 94/45 | 34/12 | 0.47a |
| Age (years), median (IQR) | 68 (62 - 74) | 67 (62 - 73) | 69 (62.3 - 75) | 0.56b |
| Smoking status (N), non/former or current smokers | 31/154 | 25/114 | 6/40 | 0.50a |
| PD-L1 status (N), ≥ 50%/1-49%/< 1%/NA | 7/9/8/161 | 6/7/6/120 | 1/2/2/41 | 0.97a |
| ECOG-PS (N), 0 - 1/≥ 2 | 143/42 | 120/19 | 23/23 | < 0.01a |
| Stage (N), IIIB/IV/recurrence | 34/139/12 | 31/98/10 | 3/41/2 | 0.03a |
| Metastatic sites (N), ≥ 2 | 93/92 | 78/61 | 15/31 | < 0.01a |
| First-line chemotherapy | ||||
| Regimen (N) | ||||
| Single/combination | 7/178 | 6/133 | 1/45 | 0.68a |
| Pemetrexed-containing | 93/92 | 64/75 | 29/17 | 0.06a |
| Bevacizumab-containing | 37/148 | 32/107 | 5/41 | 0.09a |
| Efficacy | ||||
| ORR (%) (95% CI) | 38.4 (31.3 - 45.8) | 45.3 (36.9 - 54.0) | 17.4 (7.8 - 31.4) | < 0.01a |
| DCR (%) (95% CI) | 71.9 (64.8 - 78.2) | 78.4 (70.6 - 84.9) | 52.2 (36.9 - 67.1) | < 0.01a |
| Second and further line | ||||
| Second-line (N) | 113 | 96 | 17 | < 0.01a |
| Immuno-checkpoint inhibitor (N) | 28 | 25 | 3 | 0.09a |
| Laboratory data | ||||
| NLR | ||||
| Median (IQR) | 3.3 (2.2 - 4.9) | 2.8 (2.1 - 3.9) | 6.5 (4.3 - 8.7) | < 0.01b |
| > 6 (N) | 34 | 7 | 27 | < 0.01a |
| LDH (U/L) | ||||
| Median (IQR) | 203 (170 - 257) | 189 (164.5 - 220.5) | 284.5 (239.3 - 440) | < 0.01b |
| > ULN (N) | 73 | 33 | 40 | < 0.01a |
| Albumin (g/dL) | ||||
| Median (IQR) | 3.7 (3.3 - 3.9) | 3.8 (3.6 - 4.1) | 3.1 (2.7 - 3.3) | < 0.01b |
| < 3.5 g/dL (N) | 60 | 19 | 41 | < 0.01a |
| All | Low | High | P | |
|---|---|---|---|---|
| CI: confidence interval; DCR: disease control rate; EGFR: epidermal growth factor receptor; ECOG-PS: Eastern Cooperative Oncology Group performance status; Ex: exon; IQR: interquartile range; LDH: lactate dehydrogenase; NA: not assessed; NLR: neutrophil-to-lymphocyte ratio; ORR: overall response rate; TKI: tyrosine kinase inhibitor; ULN: upper limit of normal. aFisher’s exact test; bMann-Whitney U test. | ||||
| N | 140 | 119 | 21 | |
| Backgrounds | ||||
| Sex (N), men/women | 55/85 | 46/73 | 9/12 | 0.81a |
| Age (years), median (IQR) | 73 (65 - 78) | 73 (65 - 77) | 74 (61 - 78) | 0.82b |
| Smoking status (N), non/former or current smokers | 69/71 | 60/59 | 9/12 | 0.64a |
| EGFR mutation status (N), ex19el/ex21 point/minor or compound | 69/62/9 | 59/51/9 | 10/11/ 0 | 0.52a |
| PD-L1 status (N), ≥ 50%/1-49%/< 1%/NA | 3/7/8/122 | 3/6/8/102 | 0/1/0/20 | 0.83a |
| ECOG-PS (N), 0 - 1/≥ 2 | 101/39 | 96/23 | 5/16 | < 0.01a |
| Stage (N), III/IV/recurrence | 10/96/34 | 10/75/34 | 0/21/0 | |
| Metastatic sites (N), ≥ 2 | 99 | 82 | 17 | 0.31a |
| EGFR-TKI | ||||
| Regimen (N), gefitinib/erlotinib/afatinib | 83/41/16 | 72/33/14 | 11/8/2 | 0.61a |
| Line (N), first/second or further | 108/32 | 90/29 | 18/3 | 0.41a |
| Efficacy | ||||
| ORR (%) (95% CI) | 64.3 (55.8 - 72.2) | 68.9 (59.8 - 77.1) | 38.1 (18.1 - 61.6) | 0.01a |
| DCR (%) (95% CI) | 82.9 (75.6 - 88.7) | 87.4 (80.1 - 92.8) | 57.1 (34.0 - 78.2) | < 0.01a |
| Post-EGFR-TKI therapy | ||||
| Further-line (N) | 69 | 57 | 12 | 0.48a |
| Osimeritinib (N) | 28 | 22 | 6 | 0.37a |
| Immuno-checkpoint inhibitor (N) | 6 | 5 | 1 | 1.00a |
| Laboratory data | ||||
| NLR | ||||
| Median (IQR) | 2.77 (1.93 - 4.28) | 2.52 (1.87 - 3.42) | 5.72 (3.46 - 6.93) | < 0.01b |
| > 6 (N) | 14 | 4 | 10 | < 0.01a |
| LDH | ||||
| Median (IQR) | 199.5 (176 - 240) | 193 (174 - 224) | 251 (234 - 379) | < 0.01b |
| > ULN | 49 | 30 | 19 | < 0.01a |
| Albumin (g/dL) | ||||
| Median (IQR) | 3.8 (3.3 - 4.1) | 3.9 (3.6 - 4.2) | 3.0 (2.7 - 3.2) | < 0.01b |
| < 3.5 g/dL (N) | 40 | 20 | 20 | < 0.01a |
| All | Low | High | P | |
|---|---|---|---|---|
| CI: confidence interval; DCR: disease control rate; ECOG-PS: Eastern Cooperative Oncology Group performance status; IQR: interquartile range; LDH: lactate dehydrogenase; NA: not assessed; NLR: neutrophil-to-lymphocyte ratio; nab-PTX: nanoparticle albumin-bound paclitaxel; ORR: overall response rate; PTX: paclitaxel; ULN: upper limit of normal. aFisher’s exact test; bMann-Whitney U test. | ||||
| N | 115 | 88 | 27 | |
| Backgrounds | ||||
| Sex (N), men/women | 90/25 | 71/17 | 19/8 | 0.29a |
| Age (years), median (IQR) | 71 (66 - 75.5) | 72 (66 - 76) | 70 (64.5 - 74) | 0.21b |
| Smoking status (N), non/former or current smokers | 8/107 | 7/81 | 1/26 | 0.68a |
| PD-L1 status (N), ≥ 50%/1-49%/< 1%/NA | 1/3/5/106 | 1/0/4/83 | 0/3/1/23 | 0.02a |
| ECOG-PS (N), 0 - 1/≥ 2 | 86/29 | 69/19 | 17/10 | 0.13a |
| Stage (N), IIIB/IV/recurrence | 42/59/14 | 33/41/14 | 9/18/0 | 0.04a |
| Metastatic sites (N), ≥ 2 | 34 | 28 | 6 | 0.47a |
| First-line chemotherapy | ||||
| Regimen (N) | ||||
| Single/combination | 11/104 | 9/79 | 2/25 | 1.00a |
| PTX or nab-PTX | 76 | 56 | 20 | 0.36a |
| Efficacy | ||||
| ORR (%) (95% CI) | 42.6 (33.4 - 52.2) | 44.3 (33.7 - 55.3) | 37.0 (19.4 - 57.6) | 0.66a |
| DCR (%) (95% CI) | 65.2 (55.8 - 73.9) | 64.8 (53.9 - 74.7) | 66.7 (46.0 - 83.5) | 1.00a |
| Second and further line | ||||
| Second-line (N) | 71 | 60 | 11 | 0.01a |
| Immuno-checkpoint inhibitor (N) | 11 | 6 | 5 | 0.13a |
| Laboratory data | ||||
| NLR | ||||
| Median (IQR) | 3.52 (2.46 - 5.40) | 2.97 (2.29 - 4.03) | 7.49 (5.39 - 8.65) | < 0.01b |
| > 6 (N) | 22 | 3 | 19 | < 0.01a |
| LDH | ||||
| Median (IQR) | 197 (168.5 - 230.5) | 195.5 (167.5 - 221) | 224 (173 - 278.5) | 0.07b |
| > ULN | 31 | 18 | 13 | < 0.01a |
| Albumin (g/dL) | ||||
| Median (IQR) | 3.5 (3.2 - 3.9) | 3.7 (3.4 - 3.9) | 3.1 (2.75 - 3.3) | < 0.01b |
| < 3.5 g/dL (N) | 56 | 30 | 26 | < 0.01a |
| Variable | Ad, EGFR mt (-) | P | NSCLC, EGFR mt (+) | P | SQ | P |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Ad: adenocarcinoma; CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group performance status; EGFR: epidermal growth factor receptor; HR: hazard ratio; LIPI: lung immune prognostic index; nab-PTX: nanoparticle albumin-bound paclitaxel; NSCLC: non-small cell carcinoma; SQ: squamous cell carcinoma; TKI: tyrosine kinase inhibitor. | ||||||
| Age, years | ||||||
| < 70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 70 | 1.31 (0.93 - 1.84) | 0.12 | 1.62 (0.98 - 2.69) | 0.06 | 0.95 (0.59 - 1.51) | 0.81 |
| Smoking history | ||||||
| Non-smoker | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Smoker | 1.48 (0.92 - 2.37) | 0.11 | 1.19 (0.72 - 1.99) | 0.50 | 2.06 (0.63 - 6.74) | 0.23 |
| No. of metastatic sites | ||||||
| < 2 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 2 | 0.83 (0.59 - 1.18) | 0.31 | 2.03 (1.13 - 3.66) | 0.02 | 1.51 (0.94 - 2.42) | 0.09 |
| ECOG-PS | ||||||
| 0 or 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 2 | 3.10 (2.04 - 4.70) | < 0.01 | 3.24 (1.73 - 6.06) | < 0.01 | 3.01 (1.80 - 5.04) | < 0.01 |
| Line of EGFR-TKI | ||||||
| First-line | 1 (Reference) | |||||
| Second or later line | 1.59 (0.91 - 2.78) | 0.10 | ||||
| GRIm-Score | ||||||
| Low | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| High | 2.20 (1.47 - 3.31) | < 0.01 | 1.95 (0.94 - 4.04) | 0.07 | 0.94 (0.54 - 1.65) | 0.84 |
| Variable | Ad, EGFR mt (-) | P | NSCLC, EGFR mt (+) | P | SQ | P |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Ad: adenocarcinoma; CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group performance status; EGFR: epidermal growth factor receptor; HR: hazard ratio; LIPI: lung immune prognostic index; nab-PTX: nanoparticle albumin-bound paclitaxel; NSCLC: non-small cell carcinoma; SQ: squamous cell carcinoma; TKI: tyrosine kinase inhibitor. | ||||||
| Age, years | ||||||
| < 70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 70 | 1.06 (0.77 - 1.47) | 0.72 | 1.07 (0.72 - 1.61) | 0.73 | 1.16 (0.74 - 1.83) | 0.52 |
| Smoking history | ||||||
| Non-smoker | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Smoker | 1.02 (0.66 - 1.56) | 0.94 | 1.06 (0.72 - 1.56) | 0.78 | 1.17 (0.52 - 2.62) | 0.71 |
| No. of metastatic sites | ||||||
| < 2 | 1 (Reference) | 1.41 (0.90 - 2.19) | 0.13 | 1 (Reference) | ||
| ≥ 2 | 1.01 (0.74 - 1.39) | 0.95 | 1.24 (0.78 - 1.98) | 0.36 | ||
| ECOG-PS | ||||||
| 0 or 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ≥ 2 | 1.95 (1.31 - 2.90) | < 0.01 | 1.63 (0.99 - 2.71) | 0.057 | 1.57 (0.92 - 2.69) | 0.10 |
| Bevacizumab-containing | ||||||
| Yes | 1 (Reference) | |||||
| No | 1.38 (0.93 - 2.05) | 0.12 | ||||
| Line of EGFR-TKI | ||||||
| First-line | 1 (Reference) | |||||
| Second or later line | 1.58 (0.99 - 2.52) | 0.054 | ||||
| PTX or nab-PTX-containing | ||||||
| Yes | 1 (Reference) | |||||
| No | 1.30 (0.83 - 2.04) | 0.25 | ||||
| GRIm-Score | ||||||
| Low | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| High | 1.25 (0.85 - 1.82) | 0.25 | 1.89 (1.00 - 3.55) | 0.049 | 1.10 (0.66 - 1.83) | 0.71 |