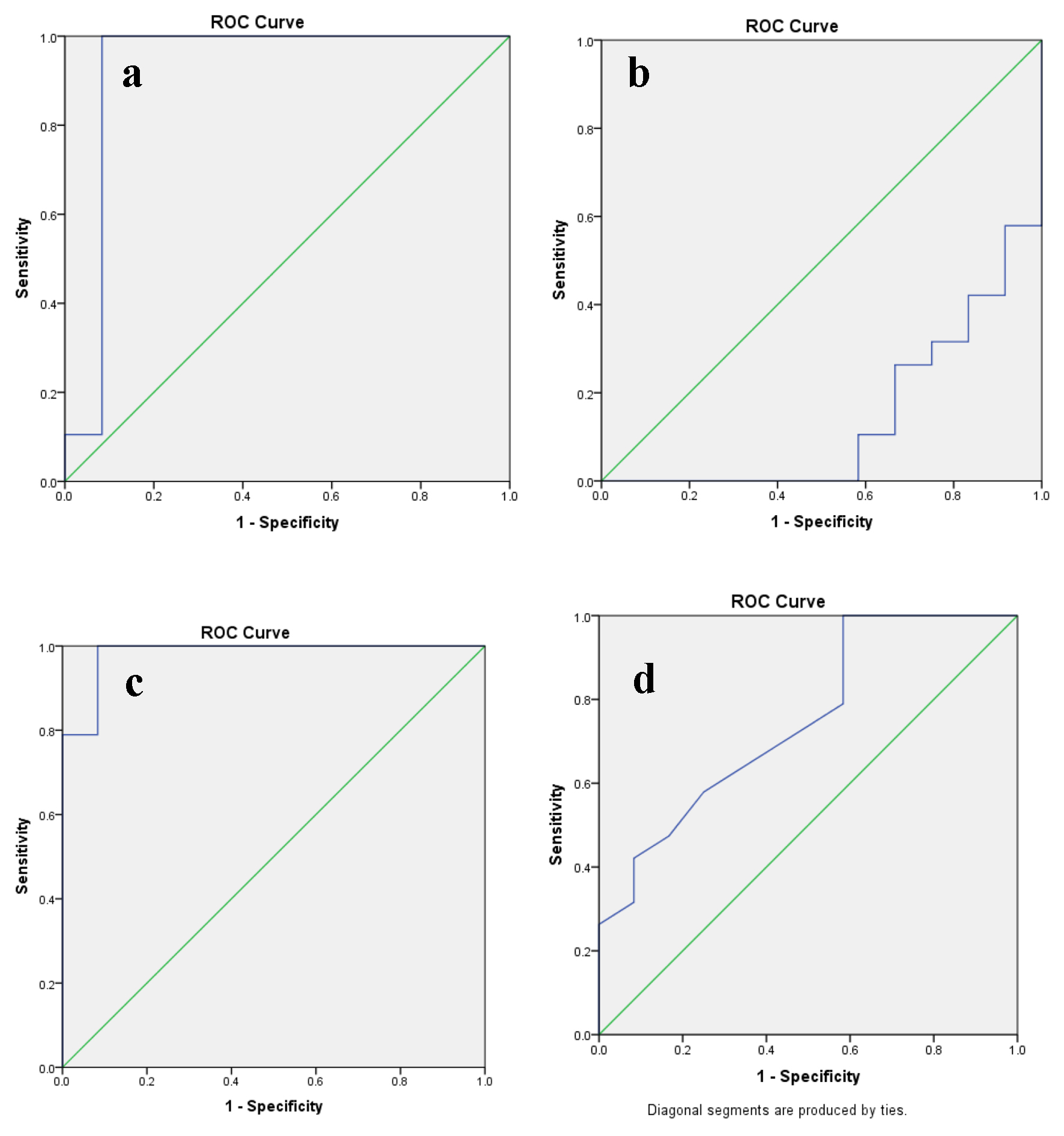
Figure 1. The predictive ability of ROC curve to determine the cut-off point for (a) PLR, (b) LMR, (c) SII index and (d) CAR in relation to disease progression. ROC: receive operating characteristic; PLR: platelet to lymphocyte ratio; LMR: lymphocyte to monocyte ratio; SII: systemic immune inflammation; CAR: C-reactive protein/albumin ratio.
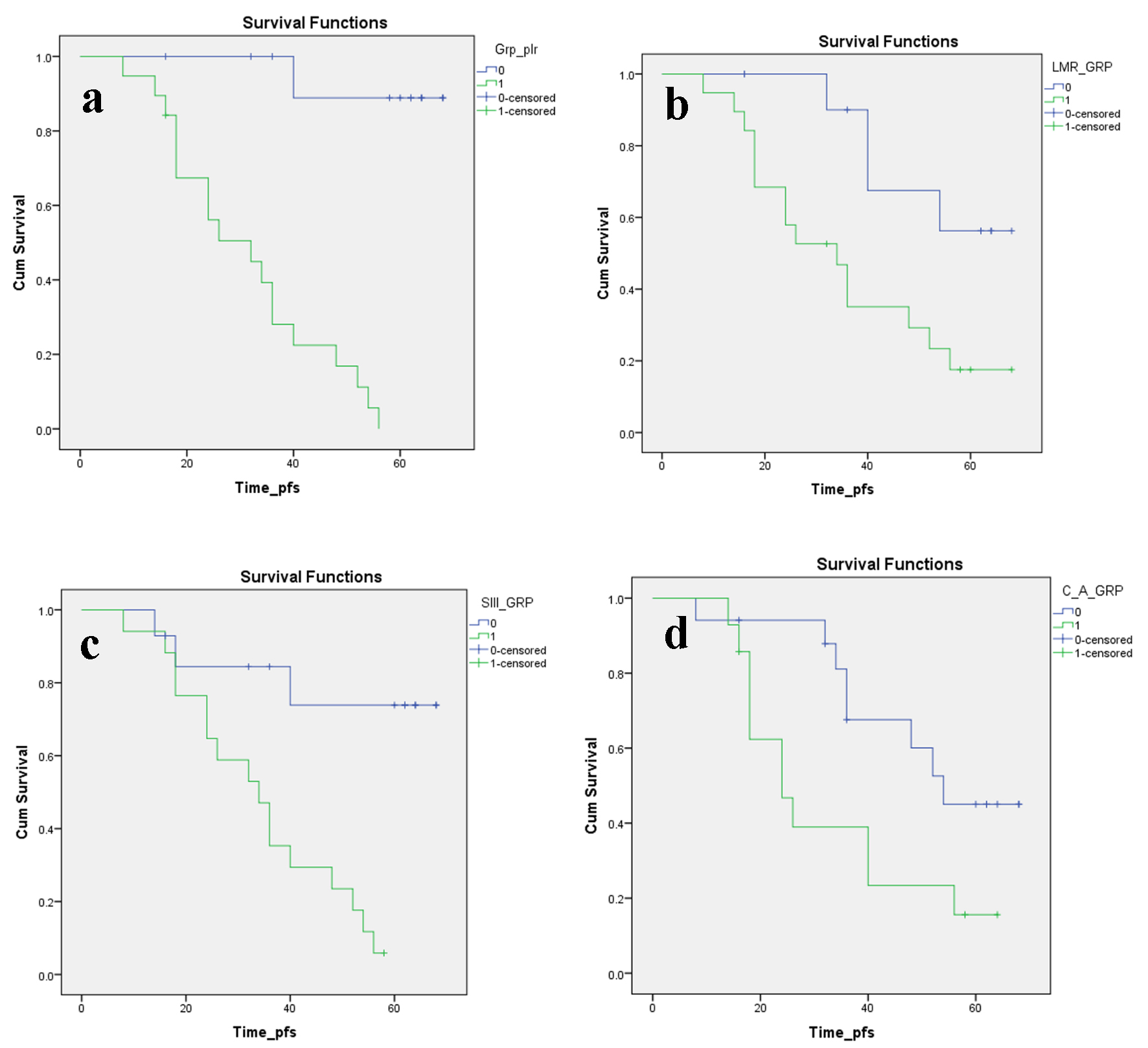
Figure 2. Kaplan-Meier plots illustrating PFS according to the (a) PLR, (b) LMR, (c) SII index and (d) CAR, respectively. PFS: progression-free survival; PLR: platelet to lymphocyte ratio; LMR: lymphocyte to monocyte ratio; SII: systemic immune inflammation; CAR: C-reactive protein/albumin ratio.
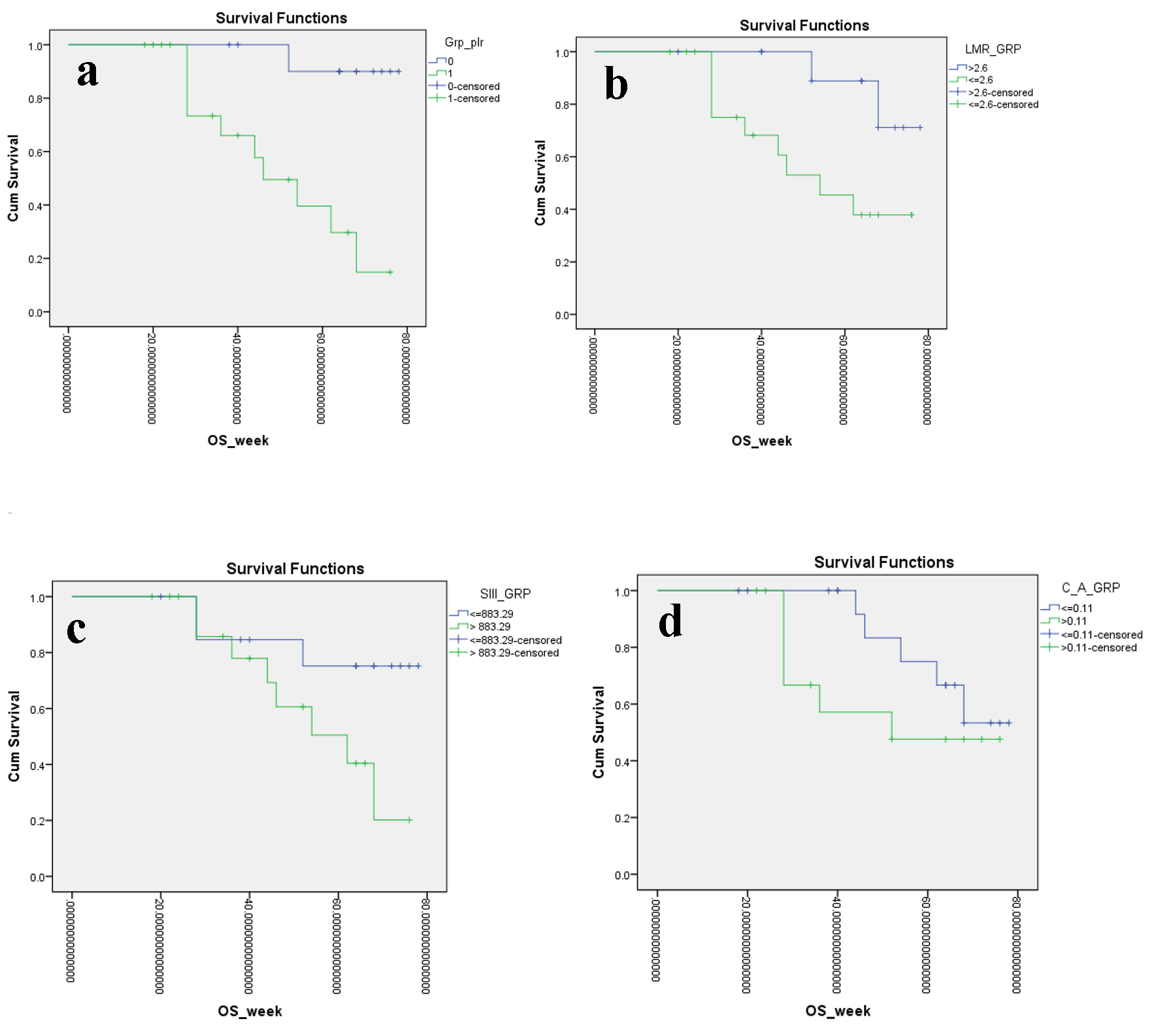
Figure 3. Kaplan-Meier plots illustrating OS according to the (a) PLR, (b) LMR, (c) SII index and (d) CAR, respectively. OS: overall survival; PLR: platelet to lymphocyte ratio; LMR: lymphocyte to monocyte ratio; SII: systemic immune inflammation; CAR: C-reactive protein/albumin ratio.


