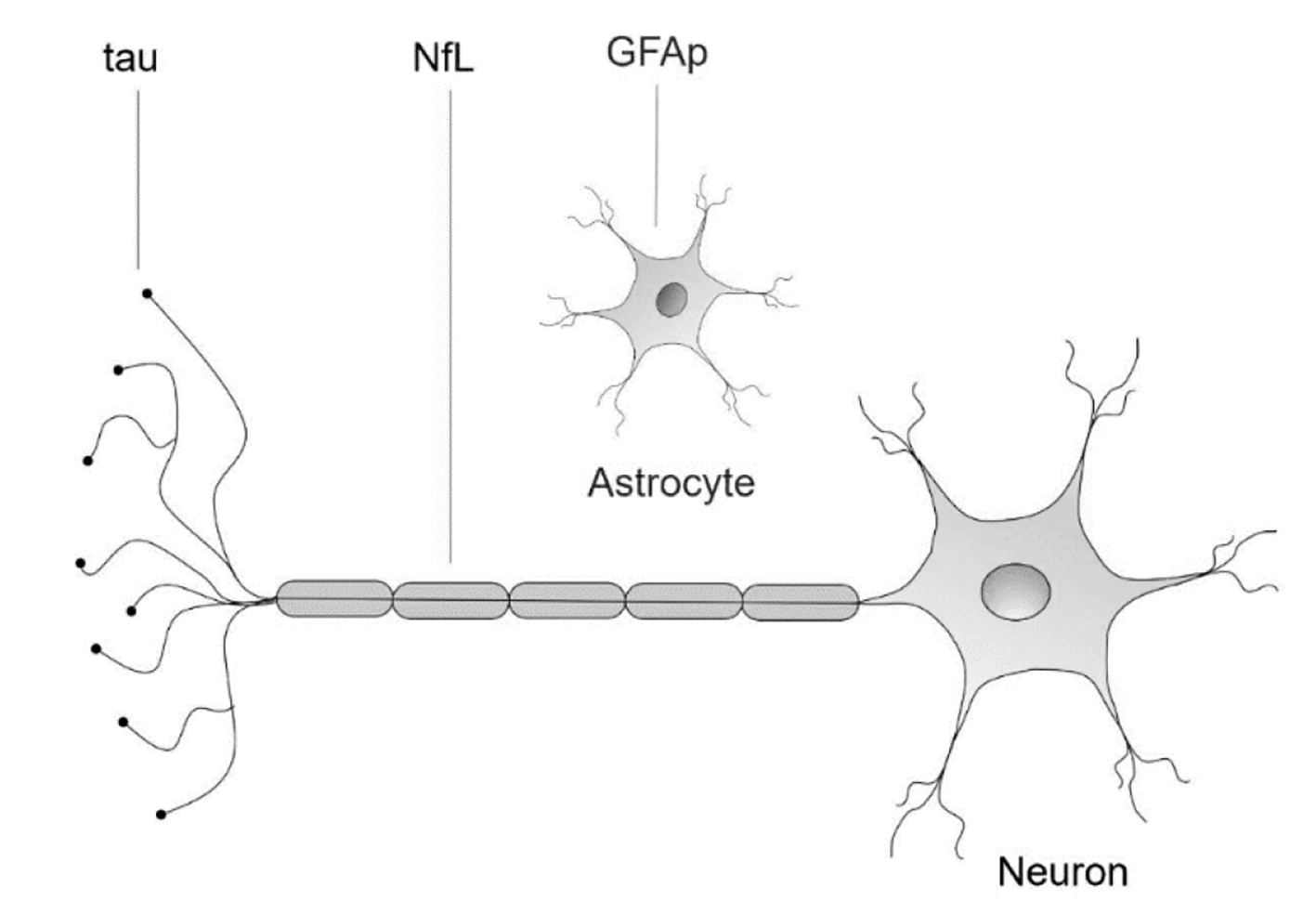
Figure 1. Neuronal and glial biomarkers. NfL: neurofilament light protein; GFAp: glial fibrillary acidic protein; tau: tau protein.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 10, Number 4-5, October 2019, pages 169-175
Serum Neurofilament Light, Glial Fibrillary Acidic Protein and Tau Are Possible Serum Biomarkers for Activity of Brain Metastases and Gliomas
Figures

Tables
| CNSPD | CNSSD | CNBM | BM | Remote BM |
|---|---|---|---|---|
| DLBCL: diffuse large B-cell lymphoma; PCL: primary central nervous system lymphoma; CML: chronic myeloid leukemia; NSCLC: non-small cell lung cancer; HER2: human epidermal growth factor receptor 2; EGFR: epidermal growth factor receptor; CNS: central nervous system; CNSPD: patients with CNS tumors with progressive disease; CNSSD: patients with CNS with stable disease; CNBM: patients with metastatic cancer with no brain metastasis; BM: patients with metastatic solid tumors with known brain metastasis; C: healthy controls; remote BM: patient without evidence of measurable disease, but with prior treatment of a metastatic brain lesion, more than 2 years before inclusion in the study. | ||||
| DLBCL | Glioblastoma | CML | Breast HR+ HER2- | Esophageal cancer HER2- |
| Mantle Cell Lymphoma | Glioblastoma | Colorectal cancer | Breast HR+ HER2- | |
| Glioblastoma | Glioblastoma | Colorectal cancer | Breast HR+ HER2- | |
| Glioblastoma | Glioma II | Colangiocarcinoma | NSCLC | |
| Glioblastoma | Glioma III | Esophageal HER2- | NSCLC | |
| Glioblastoma | HIV-PCL | Follicular Lymphoma | NSCLC | |
| Oligodendroglioma | Oligoastrocytoma | Melanoma | NSCLC EGFR mutated | |
| Oligodendroglioma | Polycythemia vera | |||
| Prostate cancer | ||||
| Group | CNSPD | CNSSD | CNBM | BM | Remote BM | C |
|---|---|---|---|---|---|---|
| NA: non-available; CNS: central nervous system; CNSPD: patients with CNS tumors with progressive disease; CNSSD: patients with CNS with stable disease; CNBM: patients with metastatic cancer with no brain metastasis; BM: patients with metastatic solid tumors with known brain metastasis; C: healthy controls; remote BM: patient without evidence of measurable disease, but with prior treatment of a metastatic brain lesion, more than 2 years before inclusion in the study. | ||||||
| No. | 8 | 7 | 9 | 7 | 1 | 4 |
| Age, median (range), years | 45.5 (42 - 74) | 58 (40 - 81) | 62 (30 - 78) | 57 (43 - 75) | 61 | 49 (36 - 62) |
| Ethnicity (%W) | 88 | 57 | 89 | 43 | 100 | 50 |
| Lines of systemic treatment, median (range) | 2 (1 - 3) | 1 (1 - 2) | 1 (1 - 3) | 1 (0 - 4) | 1 | NA |
| Radiation therapy to CNS | 6 | 6 | No | 7 | 1 | No |
| Antihistamine | No | 1 | No | No | No | No |
| Benzodiazepine | 1 | 1 | No | No | No | No |
| Memantine | No | No | No | 1 | No | No |
| Antidepressant | 2 | 1 | 4 | No | No | No |
| Antipsychotic | No | No | No | No | No | No |
| Anticonvulsant | 3 | 3 | No | No | No | No |
| Group, median (range), pg/mL | CNSPD | CNSSD | CNBM | BM | Remote BM | C |
|---|---|---|---|---|---|---|
| CNS: central nervous system; CNSPD: patients with CNS tumors with progressive disease; CNSSD: patients with CNS with stable disease; CNBM: patients with metastatic cancer with no brain metastasis; BM: patients with metastatic solid tumors with known brain metastasis; C: healthy controls; remote BM: patient without evidence of measurable disease, but with prior treatment of a metastatic brain lesion, more than 2 years before inclusion in the study. NfL: neurofilament light protein; GFAp: glial fibrillary acidic protein; tau: tau protein. | ||||||
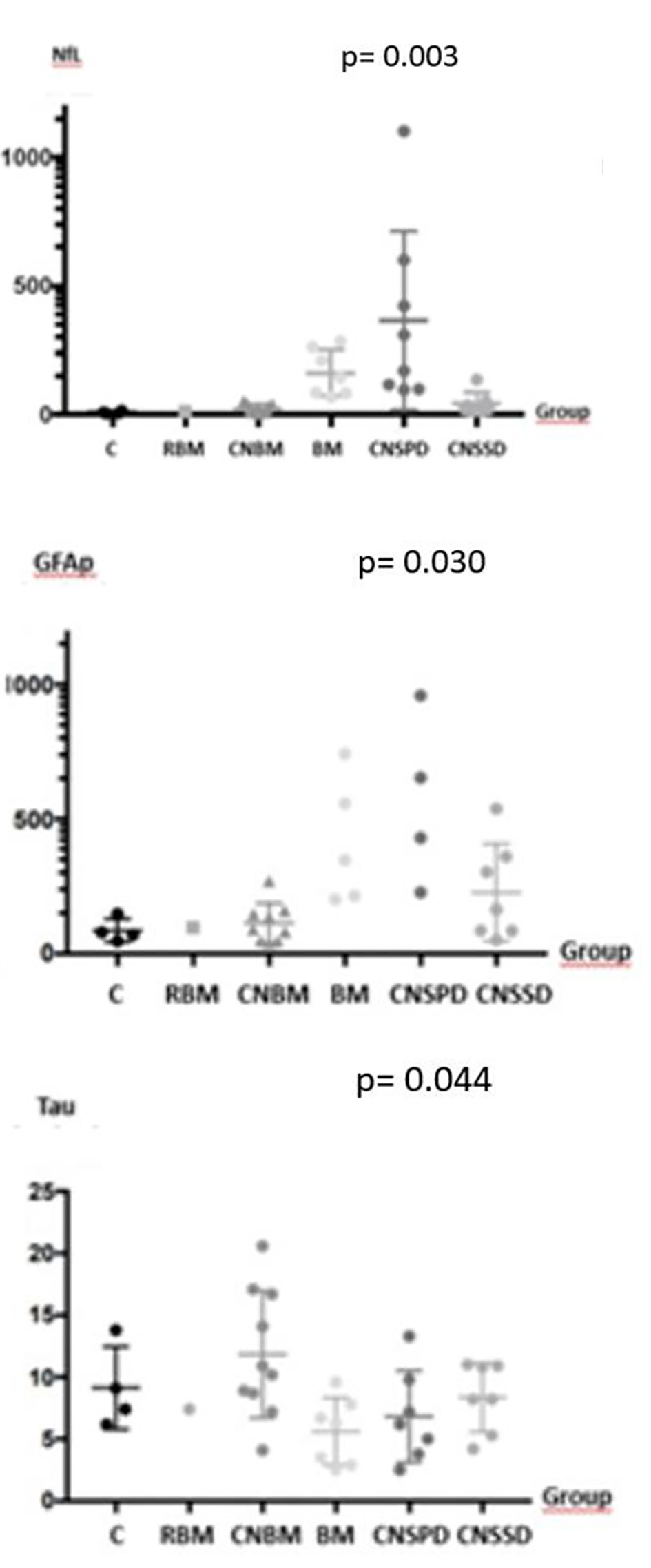
| Serum NfL | 239.4 (96.7 - 1,101.4) | 23.0 (12.6 - 135.3) | 11.8 (4.7 - 51.4) | 142.3 (67.8 - 284.9) | 13.9 | 7.2 (3.2 - 14.8) |
| Serum GFAp | 2,092.1 (227,8 - 3,435,6) | 163.1 (49.7- 538.8) | 90.2 (40.4 - 270.1) | 557.7 (201.2 - 5,972.8) | 97.1 | 74.5 (46.9 - 147.8) |
| Serum tau | 6.1 (2.5 - 13.3) | 9.5 (4.2 - 10.9) | 12.5 (4.1 - 20.6) | 6.5 (2.5 - 9.6) | 7.4 | 8.25 (6.2 - 13.8) |