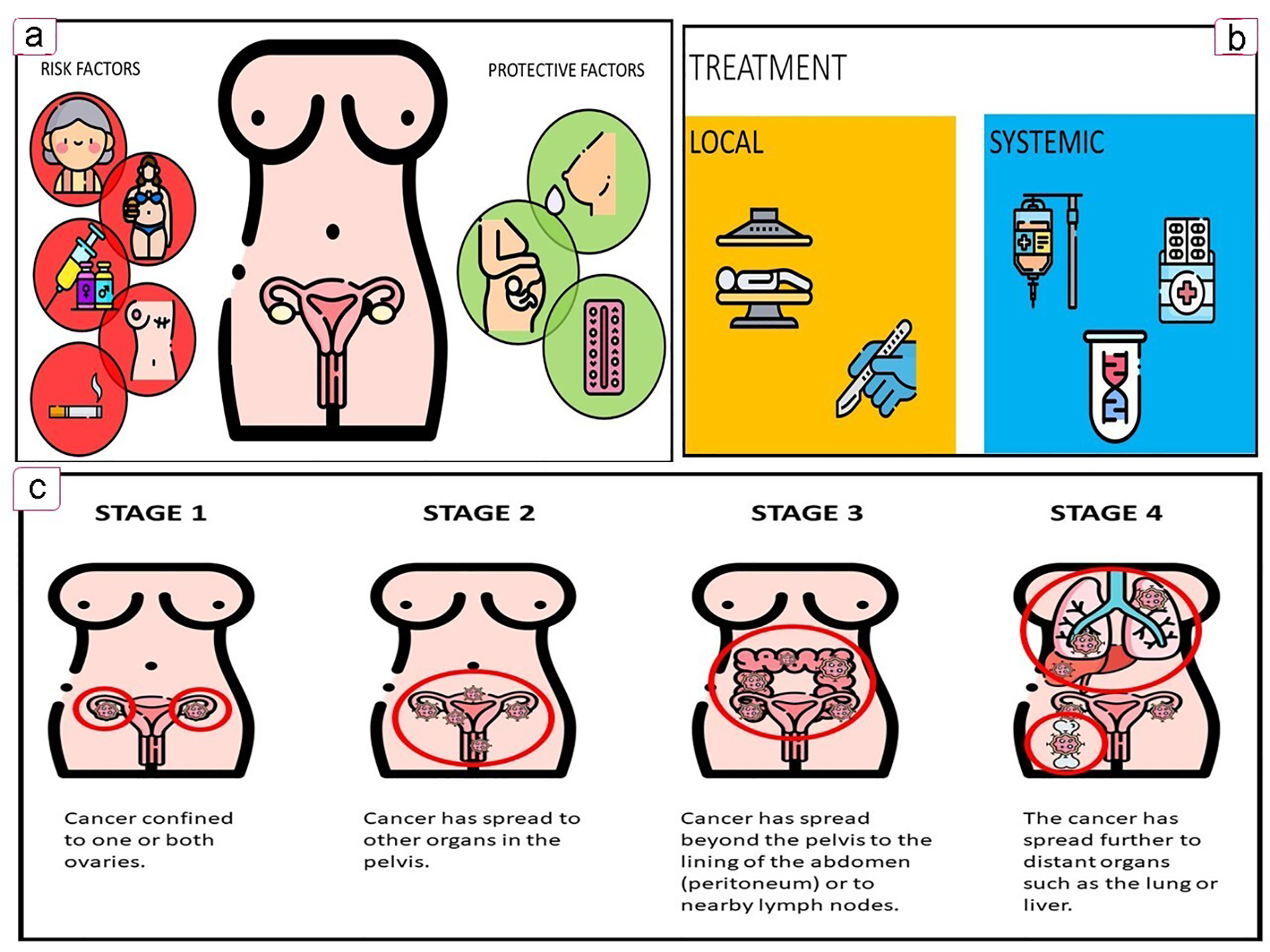
Figure 1. Key features of OC. (a) Associated factors. (b) Treatment options. (c) OC FIGO staging. OC: ovarian cancer.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 12, Number 4, August 2021, pages 85-92
Personalized Medicine in Ovarian Cancer: A Perspective From Mexico
Figure

Tables
| Worldwide | Mexico | |
|---|---|---|
| Incidence | 295,414 | 4,759 |
| Mortality | 184,799 | 2,765 |
| Prevalence | 762,663 | 12,942 |
| Year | Landmark | Reference |
|---|---|---|
| ADAMTS: A disintegrin and metalloproteinase with thrombospondin motifs; AHT: adjuvant hormone therapy; AKT: protein kinase B; ARID1A: AT-rich interaction domain 1A; CA-125: cancer antigen-125; DNA: deoxyribonucleic acid; EZH2: enhancer of Zeste homolog 2; GWAS: genome-wide association study; HE4: human epididymis protein 4; HGSC: high-grade serous ovarian cancer; miRNA: microribonucleic acid; MOC: mucinous ovarian carcinoma; mTOR: mammalian target of rapamycin; OC: ovarian cancer; OCSI: Ovarian Cancer Symptom Index; OS: overall survival; PARP: poly (ADP-ribose) polymerase; PIK3CA: p110α subunit of phosphatidylinositol 3-kinase; PM: personalized medicine; ROCA: risk of ovarian cancer algorithm; VEGF: vascular endothelial growth factor. | ||
| 2007 | VEGF-targeted therapy showed to be effective in the treatment of OC. | [9] |
| 2007 | OC screening calculated the OC risk of an individual by analyzing serial CA-125 values via ROCA and made it possible to choose the right dose of medication. | [10] |
| 2007 | The OCSI was created, anticipating being useful for early diagnosis and in this way improving personalized treatments. | [11] |
| 2008 | Biomarkers for OC, like HE4, were shown to be over-expressed in epithelial OC. Examining values of 11 markers showed that the combination of HE4 and CA-125 had the highest predictive value of this study. | [12] |
| 2008 | Screening OC patients for BRCA mutations allowed a new personalized treatment with a PARP inhibitor (AZD2281 blocks the pathway used by BRCA mutated cells to repair DNA damage). | [13] |
| 2010 | Use of bevacizumab as the leading molecular targeted agent for OC. Identification of biomarkers to select patients for bevacizumab treatment became an advance because it was well tolerated. | [14] |
| 2011 | DICER1 mutations were found in non-epithelial ovarian tumors. Aberrant miRNA processing resulted from DICER1 could be a specific feature in the development of certain types of non-epithelial OC. | [15] |
| 2010 | PIK3CA mutations could predict response to PI3K/AKT/mTOR inhibitors. PIK3CA mutations are known to be common in 12% of OC. | [16] |
| 2010 | Introduction of neoadjuvant chemotherapy in OC: right therapy to the right person. | [17] |
| 2015 | The first genetic map of how HGSC evolves in response to chemotherapy was created. At least four molecular events were associated with acquired resistance. | [18] |
| 2015 | ADAMTS mutations were found as a possible predictor of chemosensitivity in OC without BRCA1 or BRCA2 mutations. | [19] |
| 2015 | A GWAS for MOC identified three risk associations at 2q13, 2q31.1 and 19q13.2. | [20] |
| 2015 | The relation between EZH2 and ARID1A is a potentially effective treatment target for ovarian clear cell carcinoma, where < 50% of ARID1A is mutated and shows a low response to platinum-based chemotherapy. | [21] |
| 2015 | BRCAs mutations were detected in 28% of samples of OC cases. | [22] |
| 2016 | AHT after surgery becomes a personalized medicine approach for helping extend the OS of the patients with OC. | [23] |
| 2016 | Development of a cancer therapy program using integrative genomic data. Therapeutic recommendations were made after considering the genetic and genomic alterations profiles. | [24] |
| 2016 | Four commercial tools were compared to identify therapeutic recommendations for a given genetic mutation in cancers. | [25] |
| 2018 | A total of 180 BRCAs genetic variants in sporadic OC tumors were found from Mexican patients. | [26] |
| Title of the study | Recruitment countries | Research sites in Mexico | Duration | Subjects (worldwide/national) |
|---|---|---|---|---|
| OC: ovarian cancer. | ||||
| Phase III, open-label, randomized, controlled, multi-center study to assess the efficacy and safety of Olaparib monotherapy versus physician’s choice single-agent chemotherapy in the treatment of platinum-sensitive relapsed ovarian cancer in patients carrying germline BRCA 1/2 mutations. | Argentina, Belgium, Brazil, Canada, Czechia, Hungary, Israel, Italy, Republic of Korea, Mexico, Poland, Spain, and the United States of America | Oaxaca Site Management Organization, S.C., Oaxaca, Oaxaca | 6 years | 411/64 |
| Hyperthermic intraperitoneal chemotherapy in ovarian carcinoma clinical-stage IIIC and IV during interval laparotomy. Phase II study. | Mexico | National Institute of Cancerology of Mexico | 3 years | 100/100 |
| Validation of HISPANEL in Mexican patients with a high risk of hereditary breast and ovarian cancer syndrome. | Mexico | National Institute of Cancerology and Tec-Salud-Tecnologico de Monterrey | 4 years | 1,290/700 |
| Phase 3 randomized placebo-controlled double-blind study of Romiplostim for the treatment of Chemotherapy-induced Thrombocytopenia in patients receiving chemotherapy for treatment of non-small cell lung cancer (NSCLC), ovarian cancer, or breast cancer. | United States of America, Argentina, Austria, Brazil, Bulgaria, Chile, Colombia, Greece, Hungary, Mexico, Peru, Poland, Portugal, Romania, Russian Federation, Spain, Turkey, and Ukraine | Oaxaca Site Management Organization, S.C., Oaxaca, Oaxaca and not specified research sites in San Luis Potosi, San Luis Potosi and La Paz, Baja California Sur | 1 year | 162/10 |
| Drug | Manufactures | Administration | Dose | Approval | Mexico |
|---|---|---|---|---|---|
| FDA: Food and Drug Administration; OC: ovarian cancer. | |||||
| Tepadina (thiotepa) | Adienne SA | Injection | 15 mg/vial | 1959 | No |
| Platinol (cisplatin) | Hq Spclt Pharma | Injection | 75 mg/m2 | 1978 | Yes |
| Doxil (doxorubicin hydrochloride liposome) | Baxter healthcare Corp. | Injection | 2 mg/mL | 1995 | Yes |
| Gemzar (gemcitabine hydrochloride) | Lilly | Injection | 200 mg base/vial | 1996 | Yes |
| Taxol (paclitaxel) | Bristol-Myers Squibb | Injection | 175 mg/m2 | 1998 | Yes |
| Hycamtin (topotecan hydrochloride) | SmithKline Beecham Pharmaceuticals | Injection | 1.5 mg/m2 | 1998 | Yes |
| Alkeran (melphalan) | Glaxo Wellcome, Inc. | Tablets | 0.2 mg/kg | 2001 | Yes |
| Paraplatin (carboplatin aqueous solution) | Bristol-Myers Squibb | Injection | Determined by doctor | 2003 | Yes |
| Doxorubicin hydrochloride | Sun Pharm | Injection | 50 mg/m2 | 2013 | Yes |
| Lynparza (Olaparib) | AstraZeneca | Capsules, tablets | 300 mg | 2014, 2017 | Yes |
| Rubraca (Rucaparib) | Clovis Oncology, Inc. | Tablets | 600 mg | 2016 | No |
| Zejula (Niraparib) | Tesaro, Inc. | Capsules | 300 mg | 2017 | No |
| Avastin (bevacizumab) | Genetech, Inc. | Injection | 25 mg/mL | 2018 | Yes |