Figures
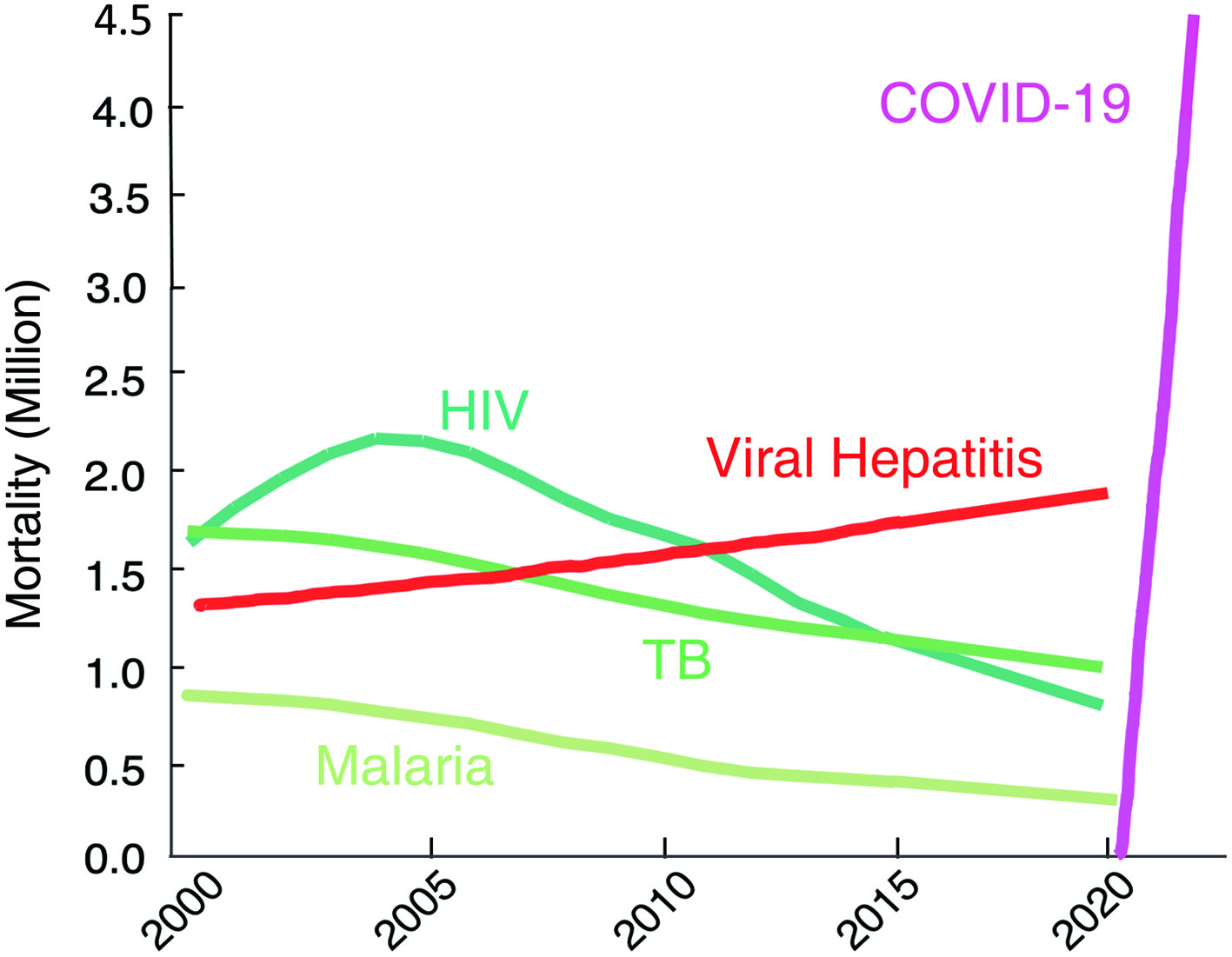
Figure 1. The death toll from coronavirus disease 2019 (COVID-19) goes far beyond that of any of human immunodeficiency virus (HIV)/tuberculosis (TB)/malaria. The number of deaths from COVID-19 surpassed by far that of fatalities of the top three communicable diseases (CDs): HIV/acquired immunodeficiency syndrome (AIDS), tuberculosis, and malaria. https://www.worldlifeexpectancy.com/world-rankings-total-deaths; https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html.
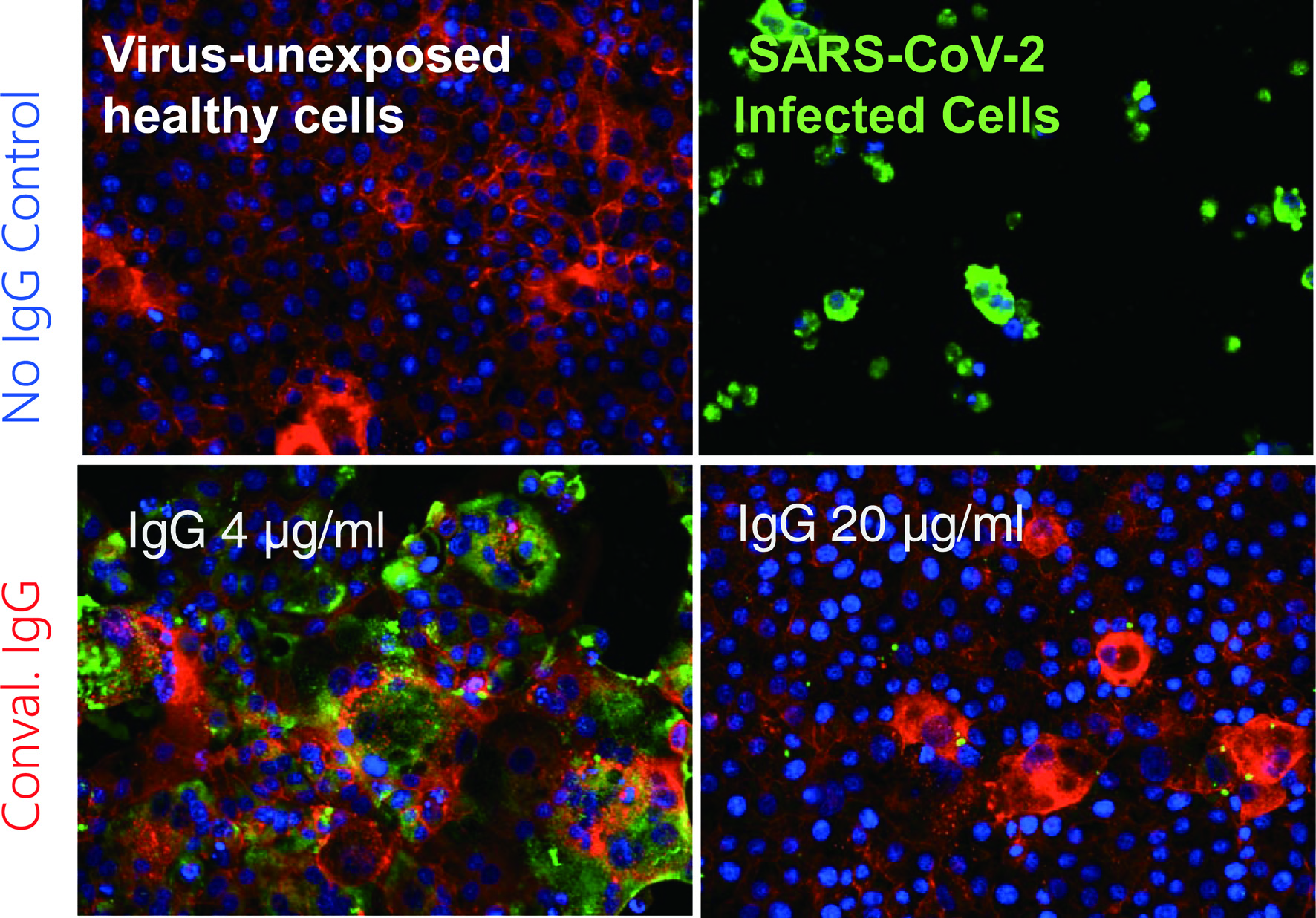
Figure 2. Coronavirus disease 2019 (COVID-19)-convalescent plasma-derived IgG completely blocks the infectivity and cytopathic effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in test tube. The method, immunocytochemistry, reveals robust cytoskeleton (filamentous actins stained in red) in virus-unexposed healthy target VeroE6TMPRSS2 cells (upper left). If infected with SARS-CoV-2, cytoskeletons are destroyed, and viral antigens are produced and stain in green and the cells are eventually destroyed (upper right). However, if the cells are exposed to SARS-CoV-2 and cultured in the presence of 4 µg/mL IgG purified from a serum sample from a COVID-19-convalescent patient, they are partially protected from the infection. In the presence of IgG 20 µg/mL, the infectivity and cytopathic effect of the virus is completely blocked. These data strongly suggest that a sufficient amount of SARS-CoV-2-neutralizing antibody can completely protect target cells from SARS-CoV-2. Cellular actin filaments, cellular nuclei, and viral antigens are stained in red (TexasRed-X), blue (DAPI), and green (Alexa Fluor 488), respectively [8].
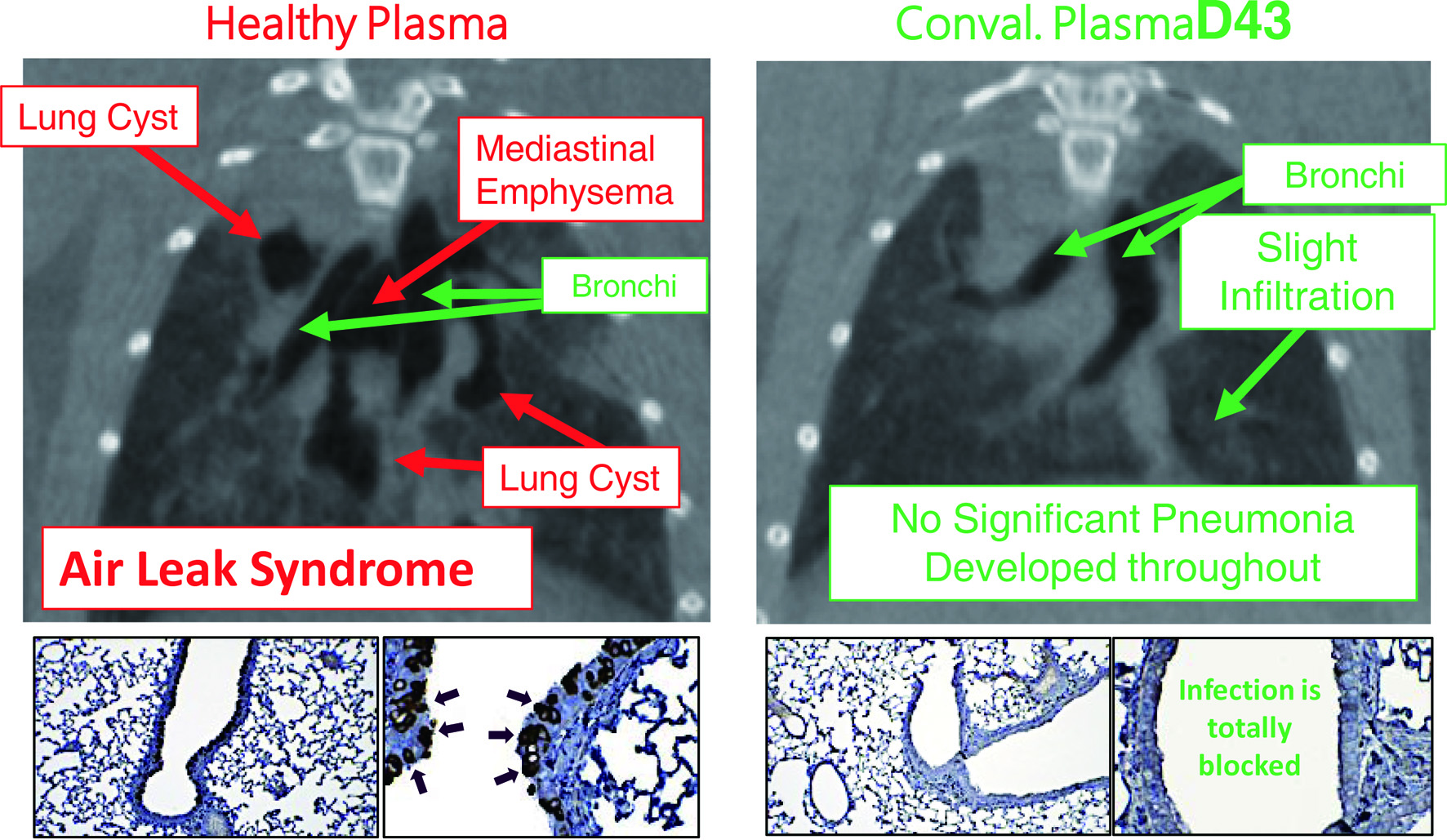
Figure 3. Hamsters receiving coronavirus disease 2019 (COVID-19)-convalescent plasma are protected from having pneumonia. Micro-computed tomography (mCT) scan was used to image the hamster lungs. For immunohistochemistry, the IgG fraction of serum from a convalescent COVID-19 individual was employed as the primary antibody and peroxidase conjugated goat polyclonal anti-human IgG antibody was used as the secondary antibody. For visualization, 3,3'-diaminobenzidine (DAB)-peroxidase enzyme reaction was performed. While Syrian hamsters, which were SARS-CoV-2-inoculated and received control healthy plasma developed severe pneumonia by day 8 following viral inoculation (upper left), hamsters which were inoculated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) but received COVID-19-convalescent human plasma (D43) were protected from infection by SARS-CoV-2 and developed no pneumonia (upper right). The two lower left insets show that many airway cells of the hamster had been infected with SARS-CoV-2 and stained in dark brown; however, the two lower right insets show no infection on the airway cells [9].
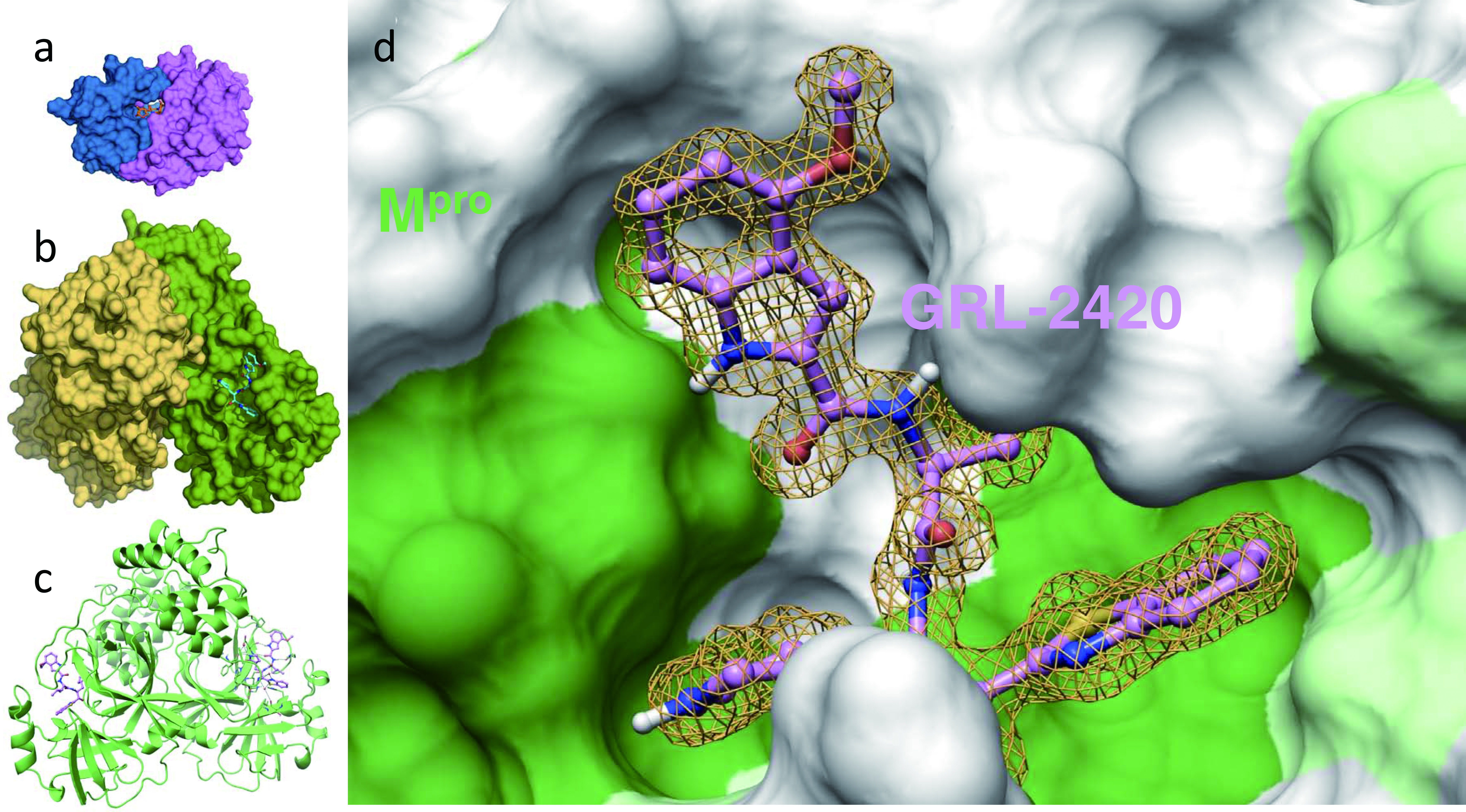
Figure 4. An Mpro inhibitor, GRL-24205h, covalently binds to Mpro , blocks Mpro’s enzymatic activity, and potently blocks severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity and replication. Inset (a) shows human immunodeficiency virus (HIV)’s protease bound by its inhibitor, darunavir, and insets (b) and (c) show SARS-CoV-2’s protease (Mpro) bound by an Mpro inhibitor, GRL-2420/5h, in the forms of surface and ribbon representations, respectively. Note that the size of Mpro is three-times larger than that of HIV’s protease. Inset d shows the hydrophobic cavity of Mpro’s enzymatic active site, into which GRL-2420/5h snugly lodges and blocks the enzymatic activity. It is of note that the algorithm of HIV/acquired immunodeficiency syndrome (AIDS) drug development serves as the basis of study of potential COVID-19 drugs [11].
Tables
Table 1. History of the US-Japan Workshops
| Date | Titles/themes | Venue |
|---|
| June 12, 2010 | The US-Japan Clinical Trials in Oncology Workshop
Career Development in Clinical Oncology | The old residence of the Japanese ambassador |
| September 13, 2013 | The 2nd US-Japan Clinical Trials in Oncology Workshop | The old residence of the Japanese ambassador |
| April 17, 2015 | The 3rd US-Japan Clinical Trials in Oncology Workshop | The old residence of the Japanese ambassador |
| June 9, 2016 | The 4th US-Japan Clinical Trials in Oncology Workshop
(Focusing on precision medicine) | Japan Information & Culture Center (JICC) |
| April 6, 2017 | The 5th US-Japan Clinical Trials in Oncology Workshop
Building your career in the field of translational oncology | Japan Agency for Medical Research and Development (AMED) Washington DC Office Conference Room |
| June 6, 2018 | The 6th US-Japan Clinical Trials in Oncology Workshop
Emergence of AI in Medicine, Good Friend or Potential Enemy | The old residence of the Japanese ambassador |
| June 5, 2019 | The 7th US-Japan Clinical Trials in Oncology Workshop
Develop Your Career | The old residence of the Japanese ambassador |
Table 2. Cancer Caused by Infectious Diseases
| Pathogens | Tumor |
|---|
| Helicobacter pylori | Gastric carcinoma, mucosa-associated lymphoid tissue (MALT) lymphoma |
| Epstein-Barr virus (EBV) | Nasopharyngeal cancer, lymphoma, gastric cancer, etc. |
| Human papilloma virus (HPV) | Cervical cancer, anal cancer, vulvar cancer, vaginal cancer, and cancer of the mid-pharynx |
| Hepatitis B virus (HBV), hepatitis C virus (HCV) | Hepatocellular carcinoma |
| Human herpes virus 8 (HHV-8, KSHV) | Kaposi’s sarcoma, primary effusion lymphoma, Castleman’s disease |
| Merkel cell polyomavirus (MCPyV) | Merkel cell carcinoma |
| Human T-cell leukemia virus type I (HTLV-1) | Adult T-cell leukemia/lymphoma (ATLL) |
| Clonorchis sinensis | Bile duct cell carcinoma |
| Schistosoma Haematobium | Bladder carcinoma |



