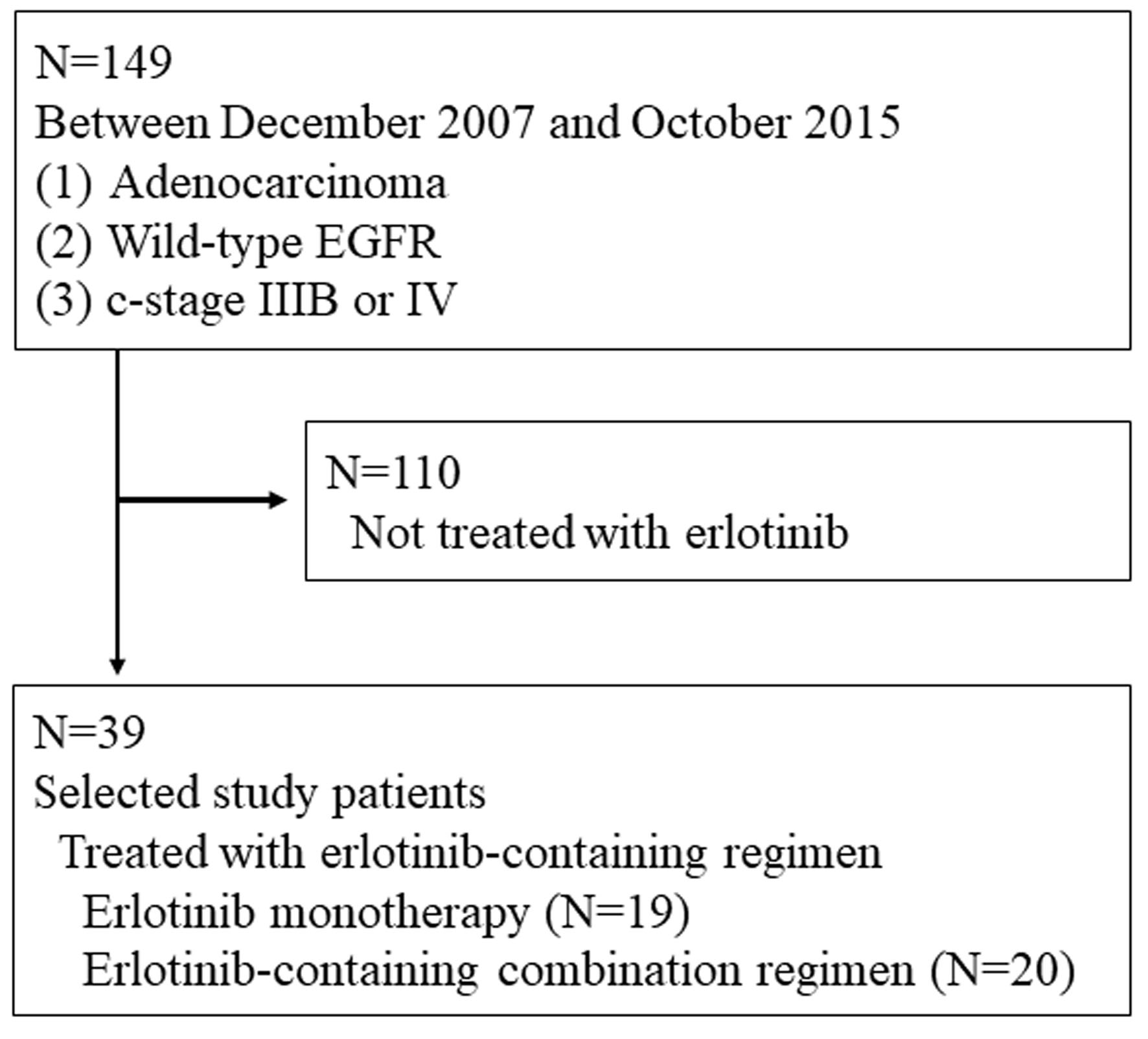
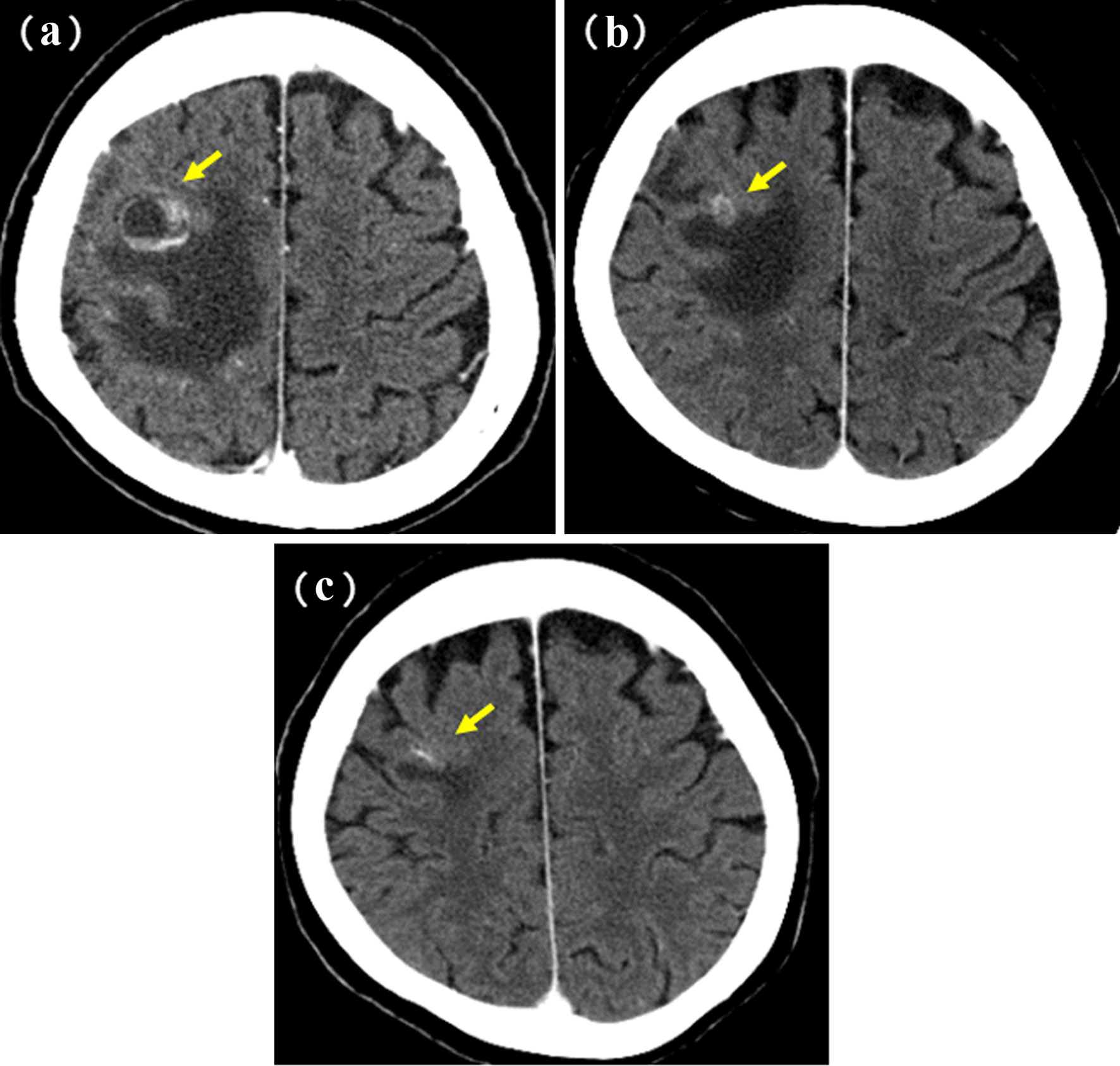
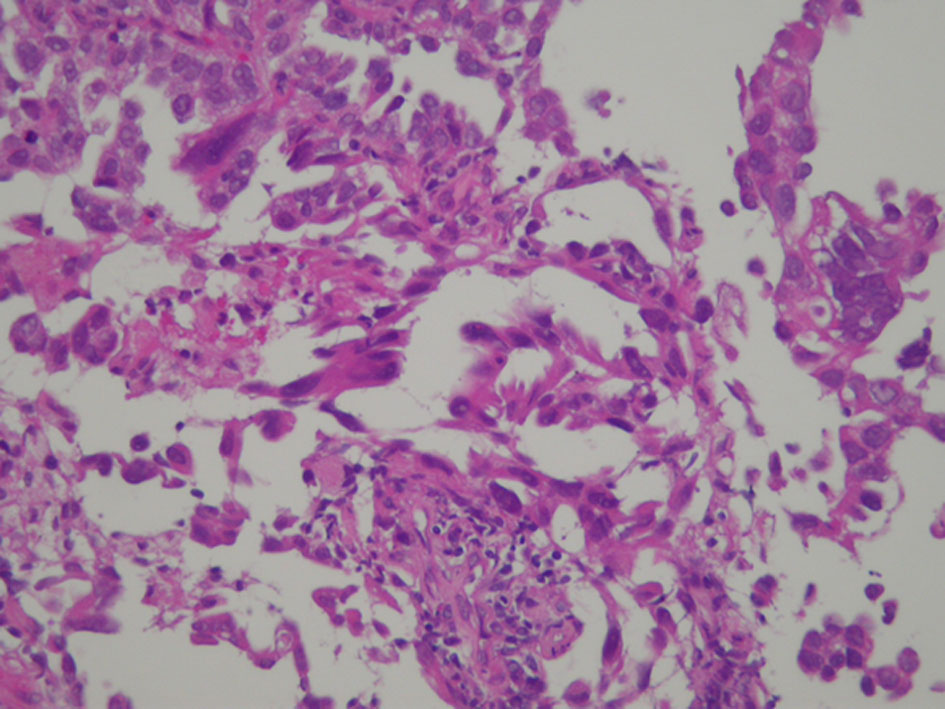
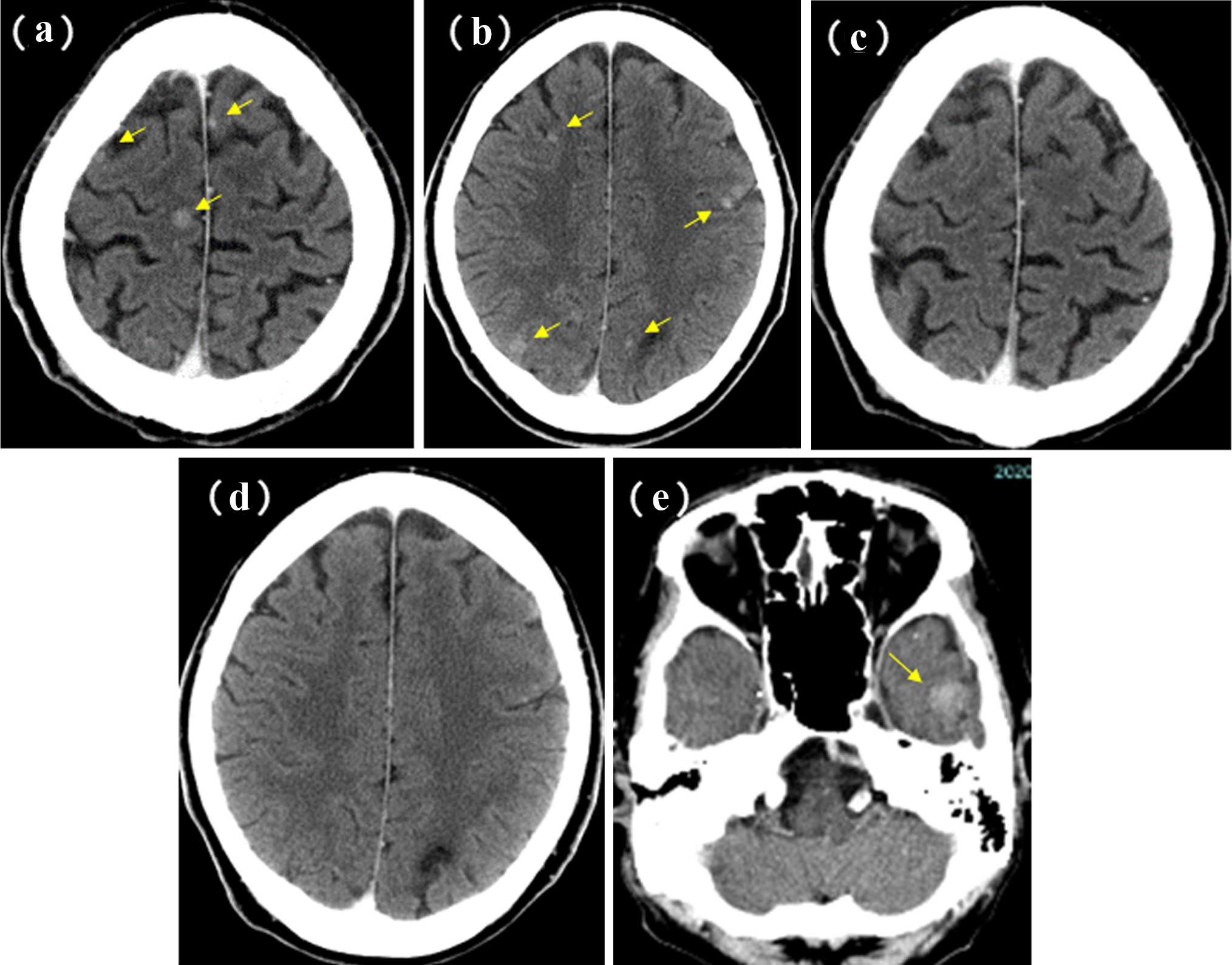
Figure 1. Patients’ selection.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 14, Number 1, February 2023, pages 101-107
Long-Term Responders to Erlotinib for Pulmonary Adenocarcinoma With Wild-Type Epidermal Growth Factor Receptor: Two Case Reports and a Single-Institutional Retrospective Study
Figures

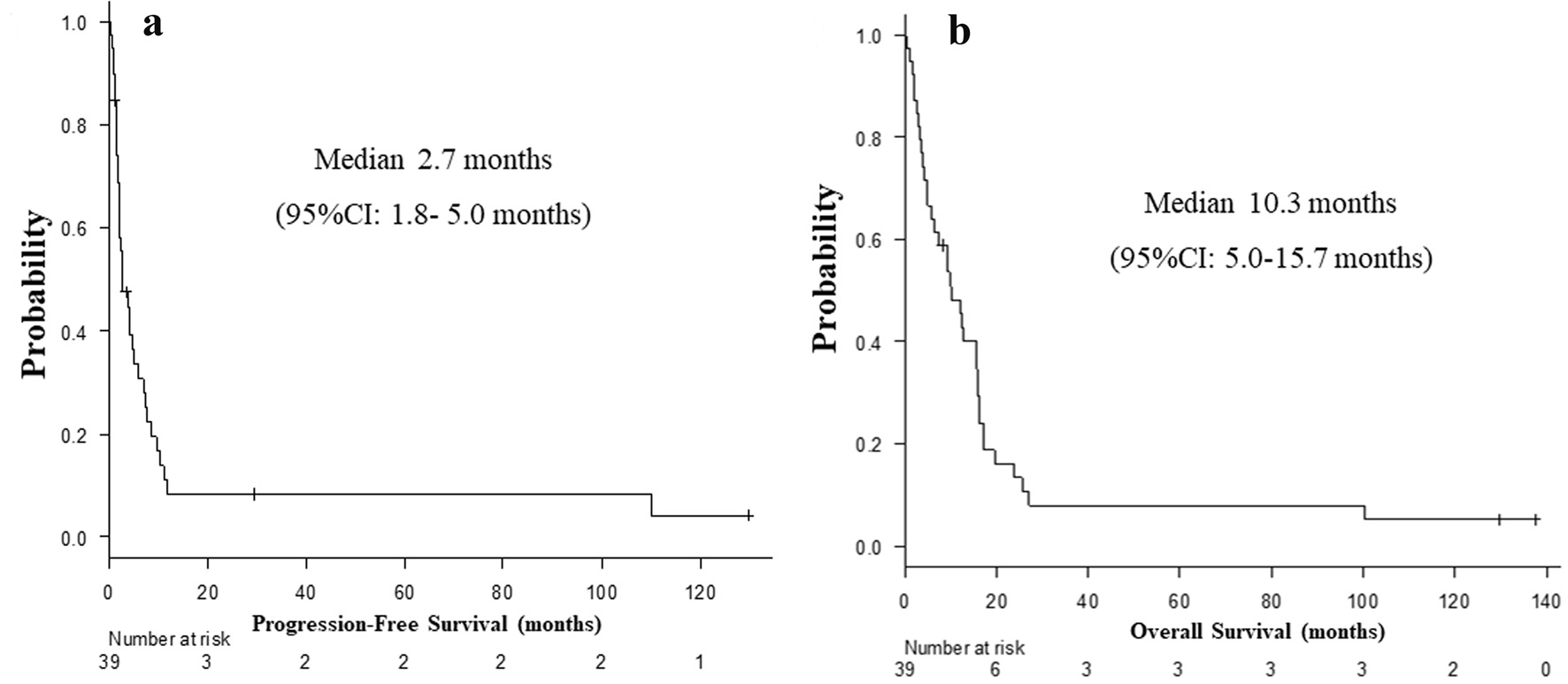
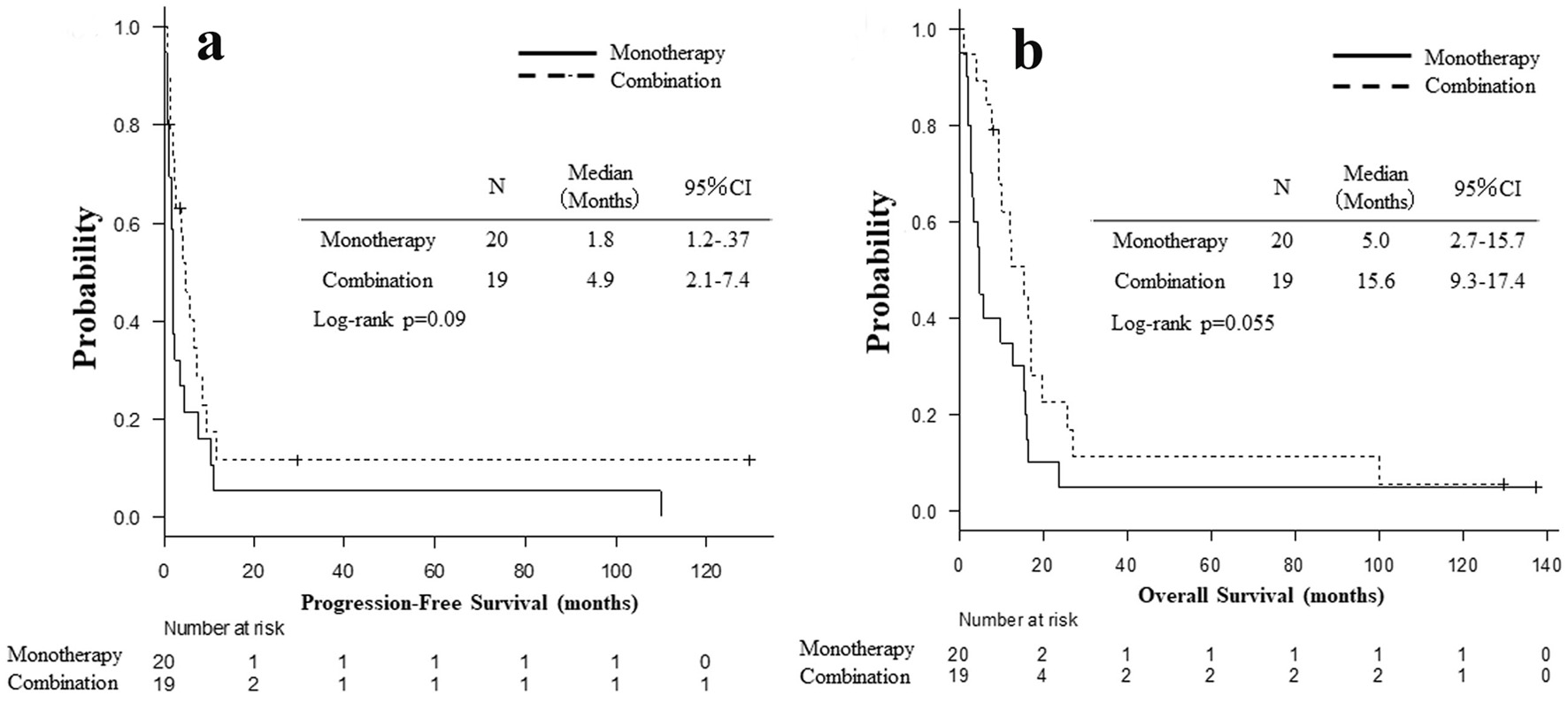
Table
| Bev: bevacizumab; CPT-11: irinotecan; CR: complete response; ECOG-PS: Eastern Cooperative Oncology Group performance status; NE: not evaluated; PEM: pemetrexed; PD: progressive disease; PR: partial response; SD: stable disease. | ||
| Backgrounds | ||
| Sex | Male/female | 25/14 |
| Age | Median (range) | 65 (42 - 80) |
| Smoking status | Non/former/current smokers | 8/13/18 |
| ECOG-PS | 0-1/2/3 | 26/12/1 |
| Distant metastases | Brain/lung/liver/bone/none | 11/19/5/7/5 |
| Treatment | ||
| Line | First/second/third/fourth | 1/30/7/1 |
| Regimen | Monotherapy | 20 |
| Combination | 19 | |
| Partner drug: PEM/CPT-11/Bev | 17/1/1 | |
| Response | CR/PR/SD/PD/NE | 2/5/10/21/1 |
| Outcomes | Alive/dead/unknown | 2/36/1 |