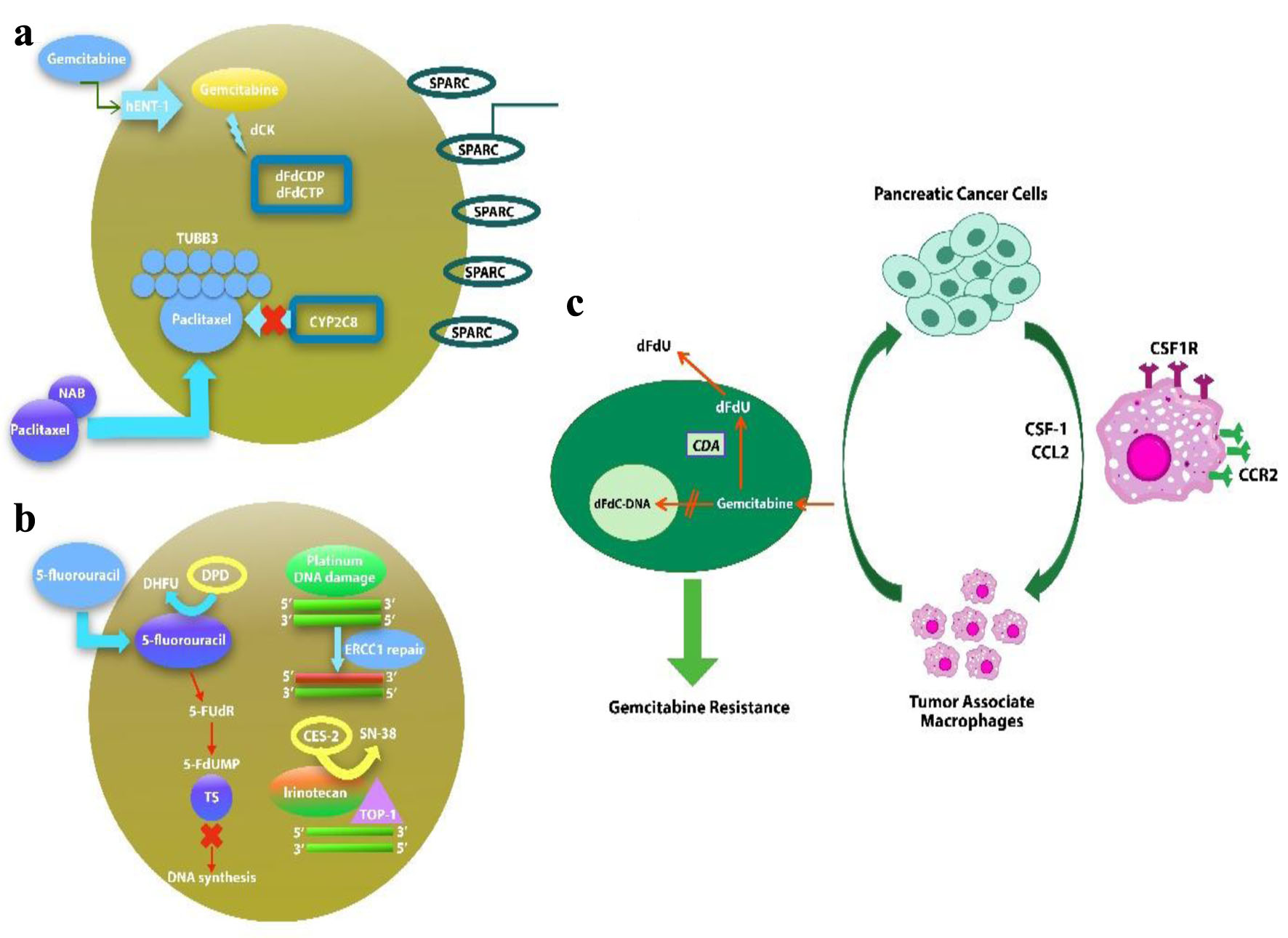
Figure 1. Potential biomarkers in nab-paclitaxel/gemcitabine (a), Folfirinox (b) regimens of drug metabolism and mechanism of action. (c) Macrophages induce gemcitabine resistance by up-regulation of CDA.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 14, Number 5, October 2023, pages 325-339
Folfirinox vs. Gemcitabine + Nab-Paclitaxel as the First-Line Treatment for Pancreatic Cancer: A Systematic Review and Meta-Analysis
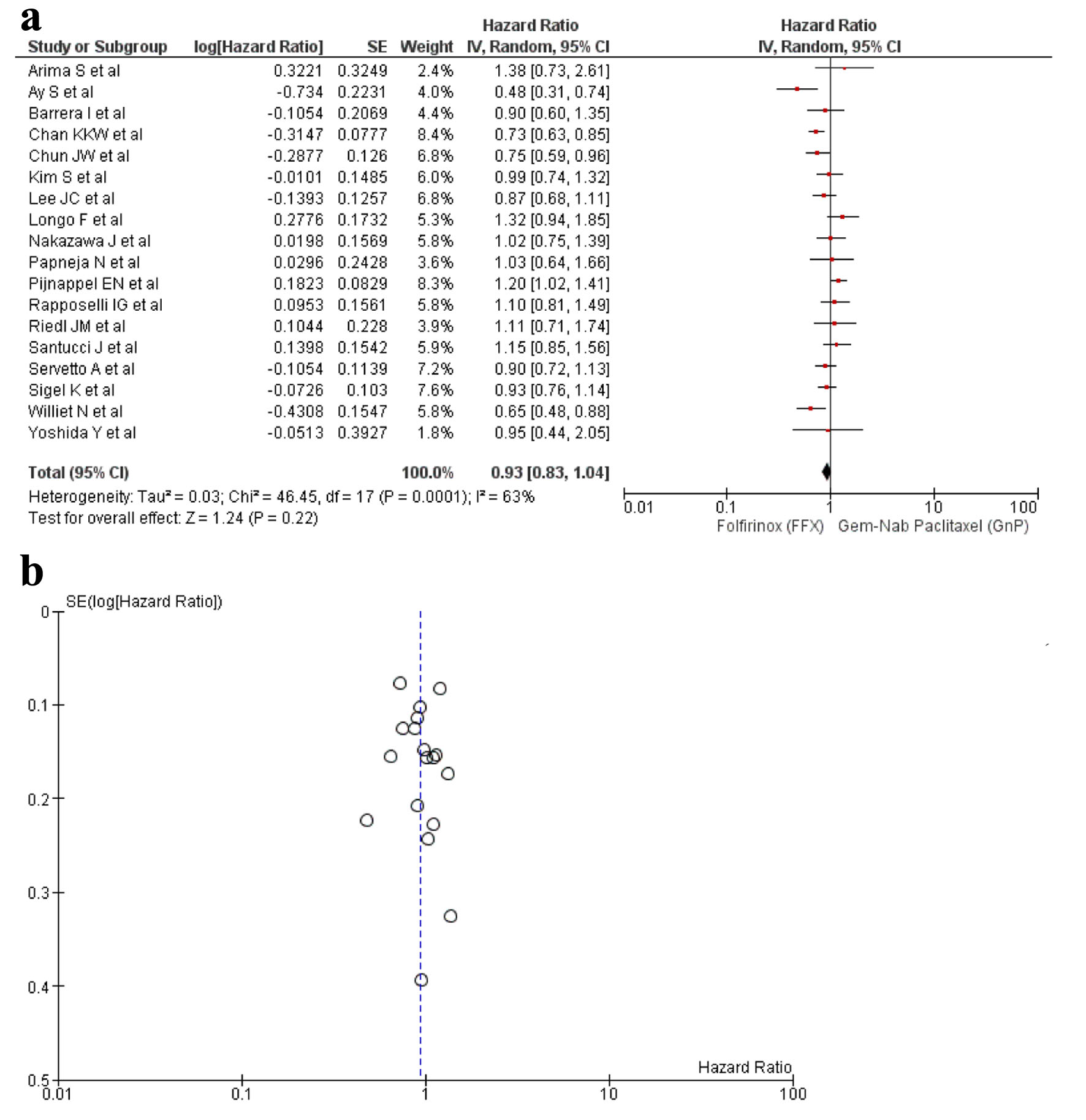
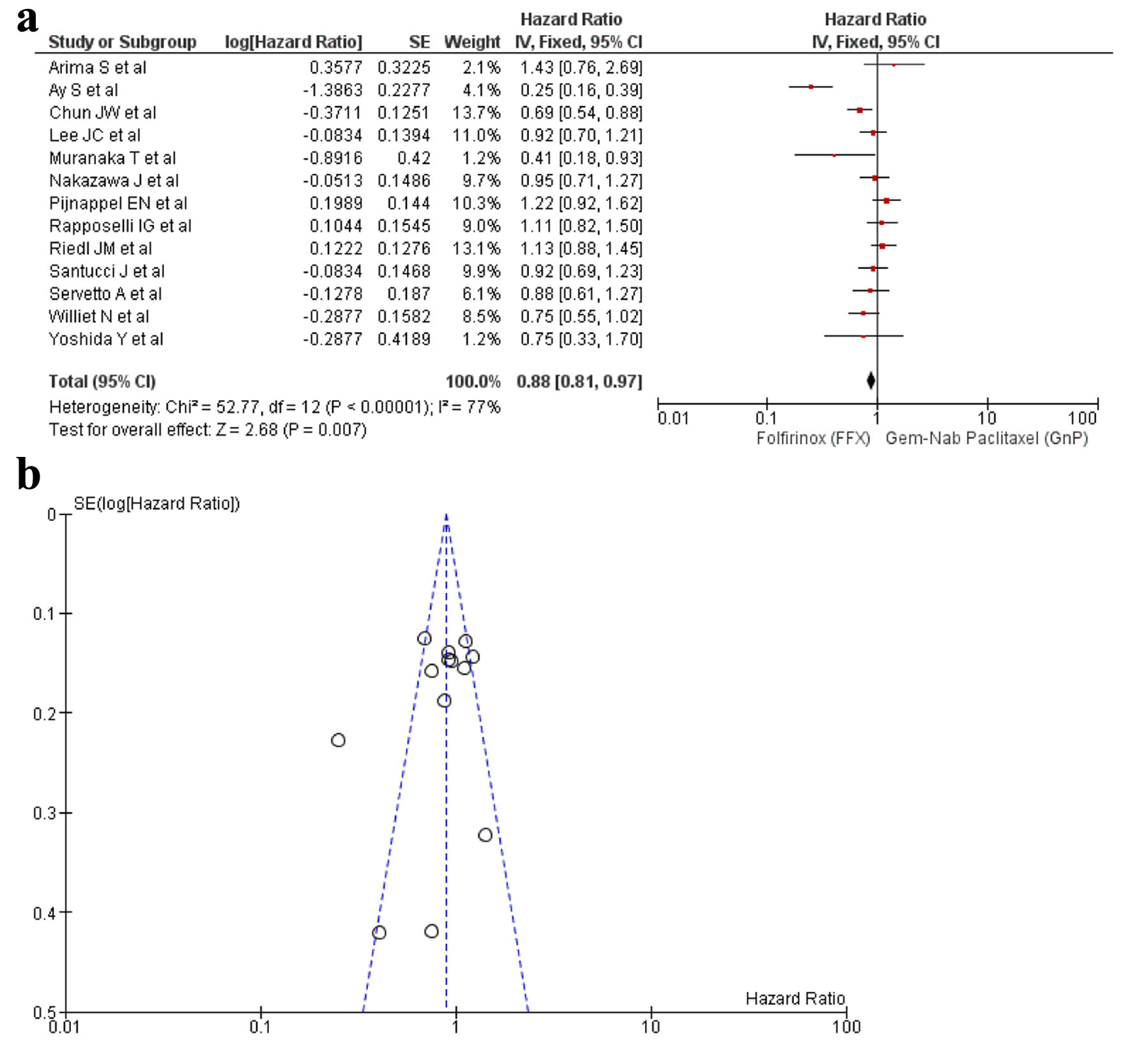
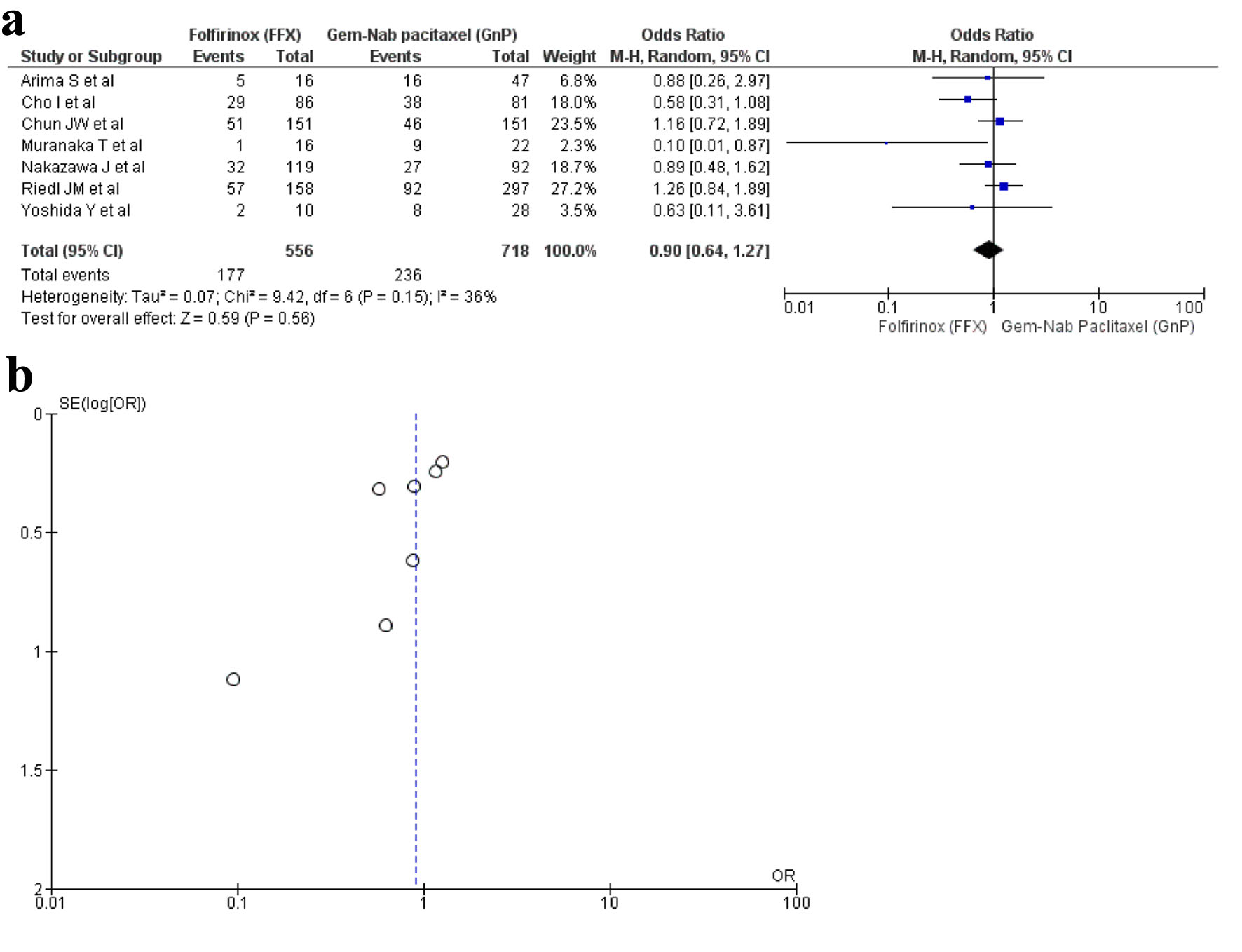
Figures

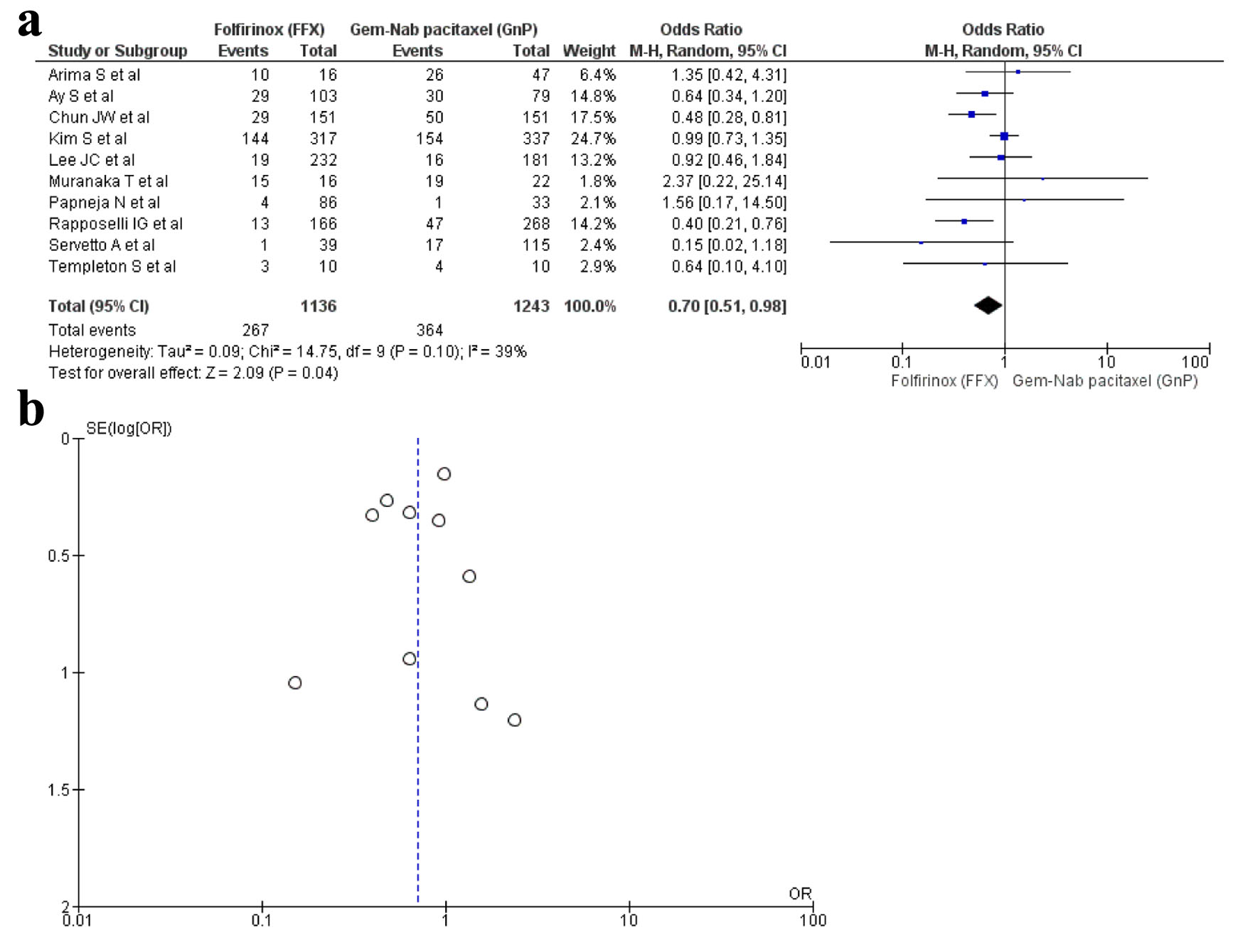
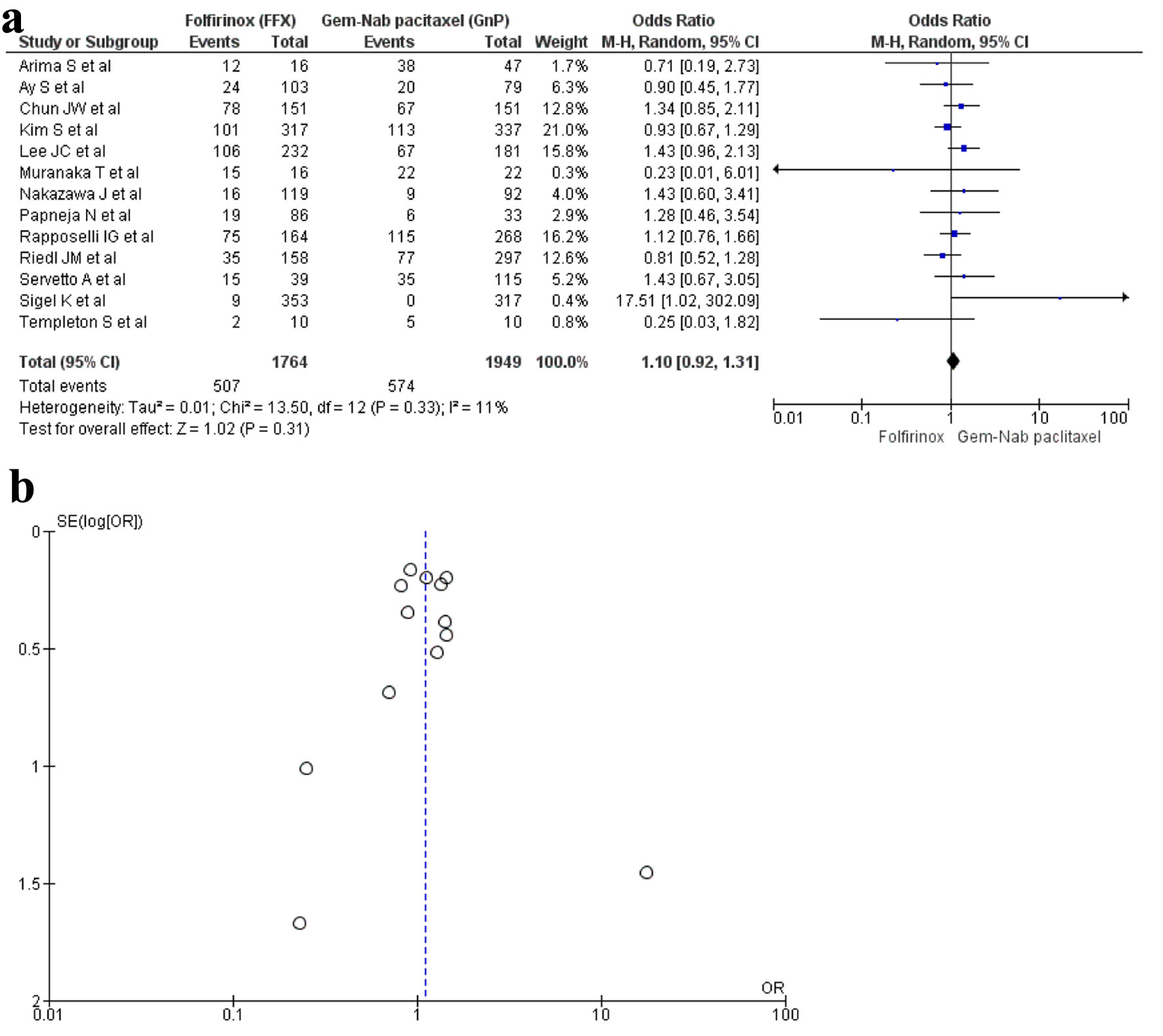
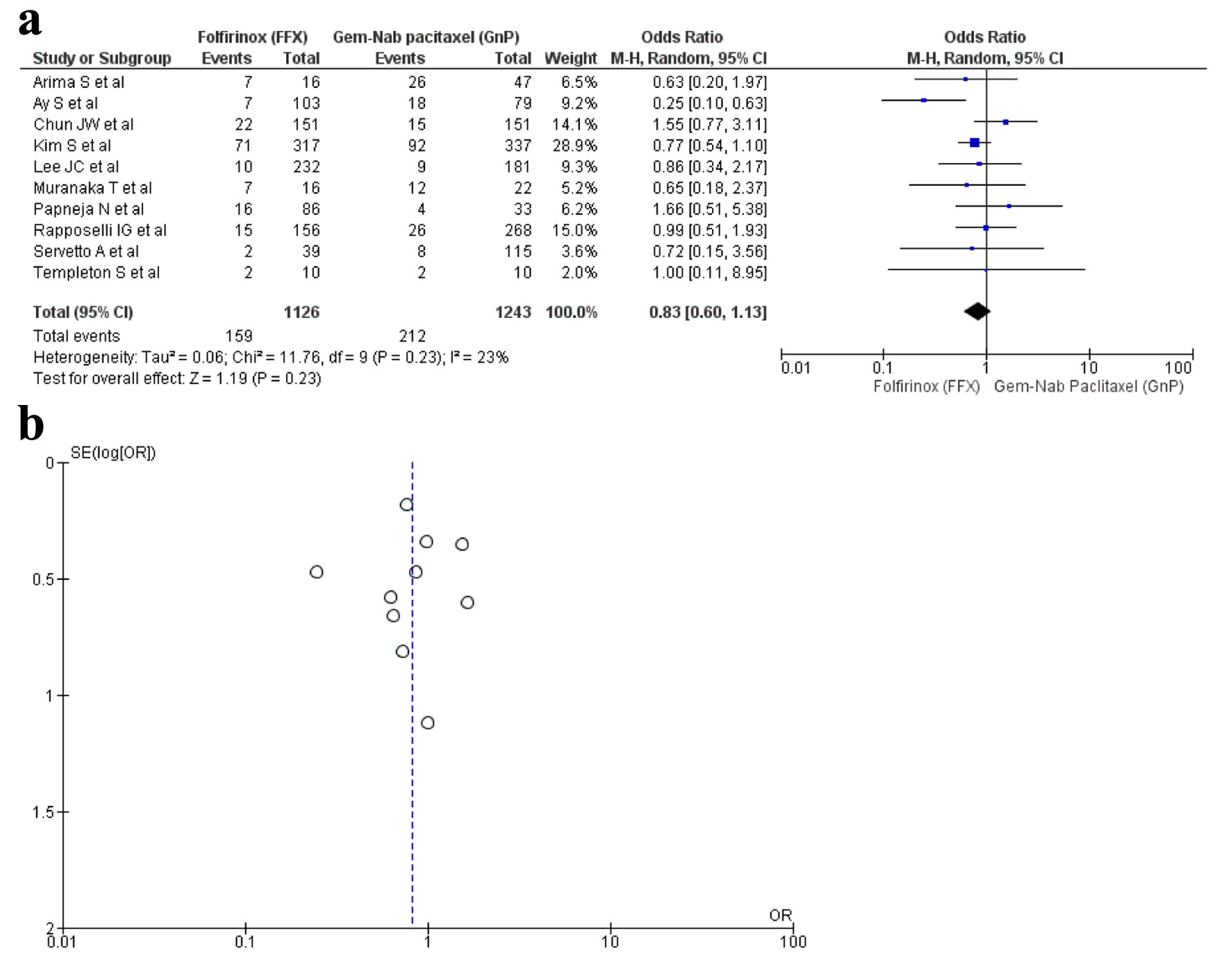
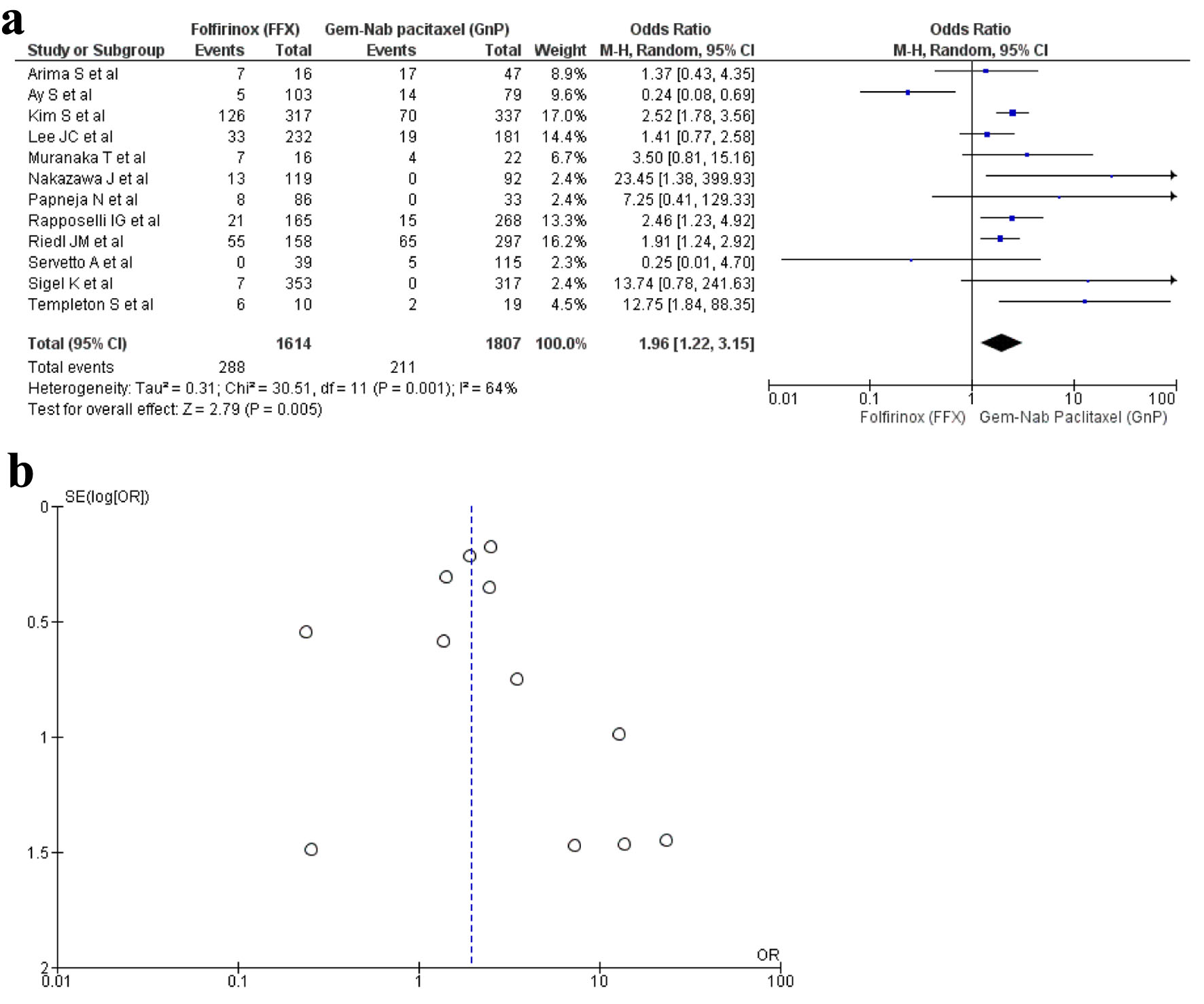
Tables
| Author | Year of publication | Country | Start time | End time | PS | Number of patients | Median age | Males (%) | Median follow-up | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| USA: United States of America; PS: performance status; NA: not applicable; GEM-NAB: gemcitabine + nab-paclitaxel. | ||||||||||
| Nakazawa et al [32] | 2019 | Japan | 2013.12 | 2018.6 | 0 - 2 | 92 | 68 | NA | 14.9 months (0.7 - 30.9) | GEM-NAB |
| 119 | 61 | NA | 13.1 months (1.3 - 40.8) | Folfirinox | ||||||
| Kim et al [29] | 2018 | USA | 2015.4 | 2015.12 | 0 - 4 | 337 | 64.59 | 64.7% | 10.7 months | GEM-NAB |
| 317 | 59.03 | 67.2% | Folfirinox | |||||||
| Muranaka et al [34] | 2017 | Japan | 2013.12 | 2015.9 | 0 - 1 | 22 | 66.5 | 54.5% | 8.3 months | GEM-NAB |
| 16 | 63 | 62.5% | 11.9 months | Folfirinox | ||||||
| Papneja et al [33] | 2019 | Canada | 2011 | 2016 | 0 - 3 | 33 | 64 | 52% | 8 months | GEM-NAB |
| 86 | 59 | 62% | Folfirinox | |||||||
| Cho et al [35] | 2018 | Korea | 2015 | NA | NA | 81 | 54 | NA | 7.9 months (1.5 - 23.4) | GEM-NAB |
| 86 | 65 | NA | Folfirinox | |||||||
| Williet et al [24] | 2019 | France | 2015.6 | 2018.6 | 0 - 2 | 109 | 68.1 | 49.5% | NA | GEM-NAB |
| 107 | 61.8 | 59.8% | Folfirinox | |||||||
| Chan et al [27] | 2019 | Canada | 2015.4 | 2017.3 | NA | 498 | 69.14 | 60.2% | NA | GEM-NAB |
| 632 | 61.83 | 54.6% | Folfirinox | |||||||
| Barrera et al [26] | 2019 | Canada | 2010 | 2018 | NA | 41 | NA | NA | NA | GEM-NAB |
| 60 | NA | NA | Folfirinox | |||||||
| 60 | NA | NA | GEM | |||||||
| Longo Munoz et al [31] | 2019 | Spain | 2011.1 | 2018.5 | NA | NA | NA | NA | NA | GEM-NAB |
| Chun et al [28] | 2021 | Korea | 2011.5 | 2019.3 | 0 - 2 | 151 | 64 | 60.3% | 33 months | GEM-NAB |
| 151 | 62 | 55.6% | Folfirinox | |||||||
| Rapposelli et al [19] | 2021 | NA | 2014.1 | 2020.12 | 0 - 2 | 268 | 62 | 61.9% | 21.8 months | GEM-NAB |
| 171 | 61 | 62% | Folfirinox | |||||||
| Lee et al [30] | 2020 | South Korea | 2011.7 | 2017.12 | 0 - 2 | 181 | 69 | 53% | NA | GEM-NAB |
| 232 | 60 | 61% | Folfirinox | |||||||
| Riedl et al [20] | 2021 | Austria | 2010.8 | 2019.10 | 0 - 1 | 297 | 70 | 59% | 26.2 months | GEM-NAB |
| 158 | 63 | 59% | Folfirinox | |||||||
| Sigel et al [23] | 2022 | NA | 2003 | 2016 | NA | 317 | 66 | 98% | NA | GEM-NAB |
| 353 | 61 | 94% | Folfirinox | |||||||
| Ay et al [17] | 2022 | NA | 2010.1 | 2020.12 | 0 - 1 | 79 | 61 | 49.4% | NA | GEM-NAB |
| 103 | 58 | 60.2% | Folfirinox | |||||||
| Santucci et al [21] | 2022 | Australia and New Zealand | 2016 | 2020 | 0 - 4 | 375 | 67 | 52.7% | 8.7 months | GEM-NAB |
| 73 | 59 | 60.3% | 6.3 months | Folfirinox | ||||||
| Yoshida et al [25] | 2022 | Japan | 2001.4 | 2017.12 | 0 - 2 | 28 | 62.5 | 57.1% | 9.4 months | GEM-NAB |
| 10 | 56.5 | 90% | Folfirinox | |||||||
| Pijnappel et al [18] | 2022 | Netherlands | 2015 | 2018 | 0 - 4 | 207 | 69 | 50% | 4.1 months | GEM-NAB |
| 1029 | 62 | 54% | Folfirinox | |||||||
| Arima et al [16] | 2021 | Japan | 2013.12 | 2017.3 | 0 - 2 | 47 | 68 | 61.7% | 14.2 months | GEM-NAB |
| 16 | 65 | 56.3% | Folfirinox | |||||||
| Templeton et al [36] | 2020 | Canada | 2011 | 2017 | 0 - 1 | 10 | 66.9 | 70% | 15 months | GEM-NAB |
| 10 | 62.6 | 70% | Folfirinox | |||||||
| Servetto et al [22] | 2022 | Italy | 2011.2 | 2020.11 | 0 - 1 | 117 | 63.7 | 57.3% | NA | GEM-NAB |
| 43 | 53.6 | 65.1% | Folfirinox | |||||||
| Author | GEM-NAB | Folfirinox | GEM-NAB | Folfirinox | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Head | Body | Tail | Head | Body | Tail | |
| GEM-NAB: gemcitabine + nab-paclitaxel. | ||||||||||
| Chun et al [28] | 91 | 60 | 84 | 67 | 33 | - | - | 37 | - | - |
| Rapposelli et al [19] | 166 | 102 | 106 | 65 | 105 | 83 | 70 | 67 | 53 | 45 |
| Lee et al [30] | 96 | 85 | 141 | 91 | 63 | 52 | 55 | 85 | 59 | 73 |
| Riedl et al [20] | 174 | 123 | 94 | 64 | 175 | 57 | 39 | 74 | 38 | 32 |
| Sigel et al [23] | 312 | 5 | 333 | 20 | - | - | - | - | - | - |
| Ay et al [17] | 39 | 40 | 62 | 41 | - | - | - | - | - | - |
| Santucci et al [21] | 198 | 178 | 44 | 29 | 209 | 80 | 70 | 40 | 14 | 16 |
| Chan et al [27] | 300 | 198 | 345 | 287 | - | - | - | - | - | - |
| Yoshida et al [25] | 16 | 12 | 9 | 1 | - | - | - | - | - | - |
| Pijnappel et al [18] | 104 | 103 | 556 | 473 | 80 | 46 | 49 | 393 | 212 | 270 |
| Arima et al [16] | 29 | 18 | 9 | 7 | - | - | - | - | - | - |
| Templeton et al [36] | 7 | 3 | 7 | 3 | 9 | 1 | - | 8 | 2 | - |
| Servetto et al [22] | 67 | 50 | 28 | 15 | 62 | - | - | 17 | - | - |
| Nakazawa et al [32] | - | - | - | - | - | - | - | - | - | - |
| Kim et al [29] | 218 | 119 | 213 | 104 | 170 | 93 | 82 | 163 | 83 | 74 |
| Muranaka et al [34] | 12 | 10 | 10 | 6 | - | - | - | - | - | - |
| Papneja et al [33] | 17 | 16 | 53 | 33 | 24 | 6 | 2 | 50 | 13 | 18 |
| Cho et al [35] | - | - | - | - | - | - | - | - | - | - |
| Williet et al [24] | 54 | 55 | 64 | 43 | 51 | 28 | 30 | 47 | 26 | 34 |
| Barrera et al [26] | - | - | - | - | - | - | - | - | - | - |
| Longo Munoz et al [31] | - | - | - | - | - | - | - | - | - | - |
| Author | Guidelines | Safety criteria |
|---|---|---|
| CTCAE: Common Terminology Criteria for Adverse Events; NA: not available. | ||
| Chun et al [28] | Good Clinical Practice guidelines and the Declaration of Helsinki | CTCAE |
| Rapposelli et al [19] | Good Clinical Practice guidelines and the Declaration of Helsinki | CTCAE |
| Lee et al [30] | NA | CTCAE |
| Riedl et al [20] | Local and National guidelines | NA |
| Sigel et al [23] | Declaration of Helsinki | NA |
| Ay et al [17] | Declaration of Helsinki | CTCAE |
| Santucci et al [21] | Declaration of Helsinki | NA |
| Chan et al [27] | NA | NA |
| Yoshida et al [25] | The Declaration of Helsinki | NA |
| Pijnappel et al [18] | Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines | NA |
| Arima et al [16] | The Declaration of Helsinki | CTCAE |
| Templeton et al [36] | ||
| Servetto et al [22] | Good Clinical Practice guidelines and the Declaration of Helsinki | CTCAE |
| Nakazawa et al [32] | NA | NA |
| Kim et al [29] | NA | NA |
| Muranaka et al [34] | The Declaration of Helsinki | CTCAE |
| Papneja et al [33] | NA | NA |
| Cho et al [35] | NA | NA |
| Williet et al [24] | The Declaration of Helsinki | National Cancer Institute CTCAE |
| Barrera et al [26] | NA | NA |
| Longo Munoz et al [31] | NA | NA |