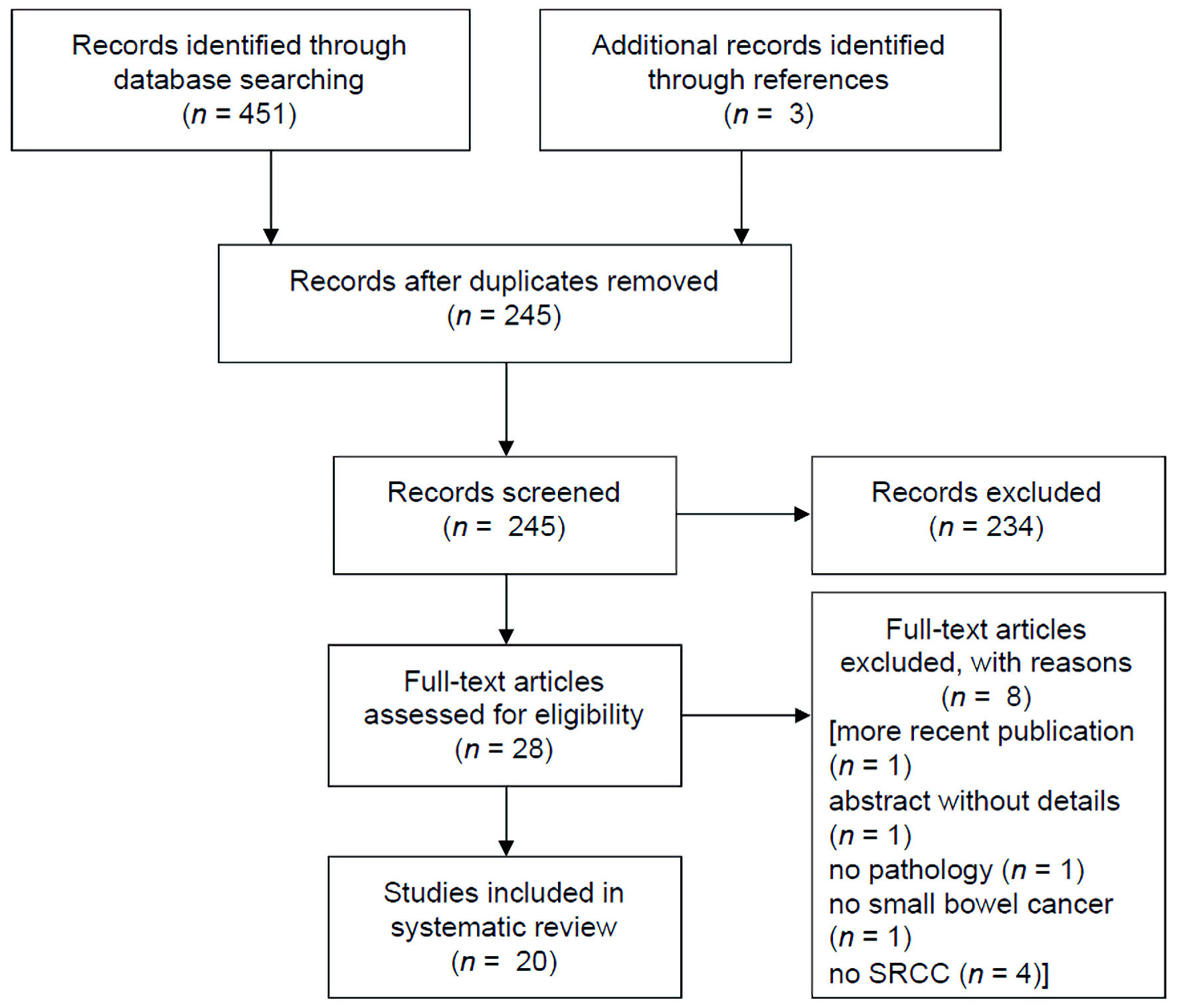
Figure 1. PRISMA study selection flow chart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 14, Number 6, December 2023, pages 447-456
Malignant Transformation of Long-Standing Ileal Crohn’s Disease Likely Favors Signet Ring Cell Adenocarcinoma Histology
Figure

Tables
| Age, sex | Crohn’s duration (years) | Surgical indication | Histology (location) | Pathological features | Postoperative treatment | Follow-up (f/u) or survival time | Country, year | Reference |
|---|---|---|---|---|---|---|---|---|
| M: male; F: female; FOLFOX: folinic acid, fluorouracil, and oxaliplatin; MMR: mismatch repair; LVI: lymphovascular invasion; PNI: perineural invasion; WT: wild type; SBA: small bowel adenocarcinoma; CD: Crohn’s disease; SRCC: signet ring cell adenocarcinoma; TI: terminal ileum; SD: standard deviation; TNF: tumor necrosis factor; mo: month; NED: no evidence of disease. | ||||||||
| 36, M | 7 | Obstruction | SRCC, adjacent high-grade dysplasia (ileum) | pT4N0M0 | - | 42 mo f/u (alive, with recurrence) | USA, 1987 | [9] |
| SBA in CD median 43 (range 33 - 72) | - | CD: SRCC 7/20 (19/20 ileum, 1/20 jejunum) | - | - | France, 2005 | [10] | ||
| SBA de novo median 68 (range 41 - 95) | De novo: SRCC 0/40 (12/40 ileum, 16/40 jejunum, 12/40 mid bowel) | |||||||
| 31, F | 15 | Complex fistulae and large abscess unresponsive to conservative treatment | SRCC (distal ileum) | 2/7 positive lymph nodes | FOLFOX | 13 mo f/u (alive) | South Korea, 2007 | [11] |
| 55, M | 31 | TI obstruction | SRCC, villous adenoma (ileum) | 16/16 positive lymph nodes, proficient MMR | Chemotherapy | - | Israel, 2011 | [12] |
| 64, M | 15 | Symptomatic CD | SRCC (ileum) | pT4N1M0 | - | - | France, 2012 | [13] |
| 40, F | 19 | Crohn’s flare with medically refractory symptoms | SRCC (TI) | pT4N0M1G3; proficient MMR; KRAS mutant | Chemotherapy | - | Germany, 2013 | [14] |
| 59, M | 28 | No surgery due to widespread metastasis on diagnosis; diagnosed due to symptoms of Crohn’s flare | SRCC (11 cm proximal to ileocecal valve) | - | Cisplatin monotherapy | 6 mo (died from disease) | Italy, 2013 | [15] |
| Three cases, mean age 50.6 (range 31 - 76) | Mean 23.4 (range 1 - 47) | - | Two cases: SRCC (small bowel) | pT4bN2aMx; + LVI | - | Recurred and died | Italy, 2014 | [16] |
| pT3NxMx; + LVI | Alive | |||||||
| One case: SRCC (at site of prior anastomosis) | pT4b.NxM1 + LVI | Recurred and died (f/u unknown) | ||||||
| 64, M | 8 | Ileal obstruction | SRCC (ileum) | Multiple mitoses, PNI, pseudopyloric metaplasia | - | - | Spain, 2014 | [17] |
| 47, F | 0, diagnosed at presentation | 2 weeks: abdominal pain, watery diarrhea, SRCC found on colonoscopy | SRCC (distal ileum) | pT3N1M0R0 | FOLFOX | 1 mo f/u (alive) | Portugal, 2016 | [18] |
| 51, M | 0, diagnosed at presentation | 6 months: bloody diarrhea, pain, tenesmus, weight loss; started on systemic therapy for Crohn’s and perforated | SRCC (ileum) | pT4N1M0R0 | FOLFOX | 1 mo f/u (alive) | Portugal, 2016 | [18] |
| 58, F | 0, diagnosed at presentation | TI stenosis without complete obstruction, SRCC found on colonoscopy biopsies | SRCC (TI, invading adjacent sigmoid) | PNI, pseudopyloric metaplasia, pT4N2M0 | FOLFOX | 5 mo f/u (alive) | Portugal, 2018 | [19] |
| 46, M | 15 | Obstruction | SRCC, villous dysplasia (distal ileum) | G3, T4N1M1; proficient MMR, BRAF/KRAS WT | - | 11 mo (died from disease) | France, 2017 | [20] |
| 64, M | 32 | Obstructive symptoms and low-grade dysplasia on preoperative colonoscopy biopsy | SRCC, adenomatous dysplasia (distal ileum) | G3, T4N0M0; germline mutation of HLML1 and PMS2, BRAF/KRAS WT | - | 66 mo f/u (alive) | France, 2017 | [20] |
| 63, M | 32 | Symptomatic CD refractory to steroids and TNF blockade | SRCC (ileum), with carcinomatosis | pT4aN2M1; 12/15 positive nodes; proficient MMR; + LVI, + PNI | - | - | USA, 2018 | [21] |
| 68, M | 0, diagnosed at presentation | Perforation | SRCC (40 cm from ileocecal valve) | pT4N0M0 | Referred to medical oncology | 9 mo f/u (alive, NED) | Algeria, 2019 | [22] |
| 44, F | - | Hematochezia, SRCC diagnosed on endoscopy | SRCC (TI) | pT4aN1M0; RAS/RAF WT | FOLFOX | - | USA, 2019 | [23] |
| 67, M | 44 | SRCC on colonoscopy biopsy after medically managed flare | SRCC, tubulovillous adenoma (distal ileum) | G3, pT2N1M0 | FOLFOX | - | USA, 2020 | [24] |
| 64, M | 3 | Acute abdomen, TI perforation | SRCC (distal ileum) | G2, pT4N1Mx. 9/25 positive lymph nodes | Chemotherapy | - | Romania, 2021 | [25] |
| 53.73 ± 14.52 (mean ± SD); 4/15, F | - | 11/15 cases CD | SRCC, 66.7% ileum, 20% duodenum, 13.3% jejunum | pT3 in 46.7% and pT4 in 53.3%; positive nodes; 93.3% proficient MMR; 53.3%; + LVI 93.3%; + PNI 93.3% | - | - | Italy, 2022 | [26] |
| 68, F | 0, diagnosed at presentation | Newly diagnosed Crohn’s, colonoscopy biopsy positive for cancer | SRCC (arising from tubulovillous adenoma of TI) | pT4 (no other information) | FOLFOX | 3 mo f/u (alive) | USA, 2022 | [27] |
| 41, M | Suspected new diagnosis of Crohn’s | Anemia, weight loss, fistula to buttock | SRCC (TI) | pT4bN2b, MMR proficient; + LVI; + PNI; KRAS WT, negative for NTRK, BRAF, TNB, HER-2, PIK3 and PDL1 | FOLFOX | 3 mo f/u (alive) | USA, 2022 | [28] |
| Location | Small bowel | Duodenum | Jejunum | Ileum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma | Signet ring | P value | Adenocarcinoma | Signet ring | P value | Adenocarcinoma | Signet ring | P value | Adenocarcinoma | Signet ring | P value | |
| Incidence rates per 1 million. CSS: cause-specific survival; CI: confidence interval. | ||||||||||||
| N (%) | 6,111 (100) | 327 (100) | - | 3,702 (60.6) | 151 (46.2) | - | 959 (15.7) | 29 (8.9) | - | 670 (11.0) | 92 (28.1) | - |
| Age (years) (%) | ||||||||||||
| 0 - 14 | 0 (0) | 0 (0) | < 0.001 | 0 (0) | 0 (0) | 0.02 | 0 (0) | 0 (0) | 0.35 | 0 (0) | 0 (0) | 0.13 |
| 15 - 29 | 42 (0.7) | 5 (1.5) | 17 (0.5) | 2 (1.3) | 13 (1.4) | 1 (3.4) | 8 (1.2) | 2 (2.2) | ||||
| 30 - 49 | 794 (13.0) | 55 (16.8) | 344 (9.3) | 19 (12.6) | 206 (21.5) | 8 (27.6) | 106 (15.8) | 17 (18.5) | ||||
| 50 - 69 | 2,533 (41.4) | 157 (48.0) | 1,416 (38.2) | 71 (47.0) | 476 (49.6) | 9 (31.0) | 295 (44.0) | 50 (54.3) | ||||
| 70 - 85 | 2,129 (34.8) | 90 (27.5) | 1,465 (39.6) | 47 (31.1) | 221 (23.0) | 9 (31.0) | 197 (29.4) | 18 (19.6) | ||||
| > 85 | 613 (10.0) | 20 (6.1) | 460 (12.4) | 12 (7.9) | 43 (4.5) | 2 (6.9) | 64 (9.6) | 5 (5.4) | ||||
| Mean (SD) | 66.4 (14.5) | 62.7 (14.3) | < 0.001 | 68.9 (13.8) | 64.7 (14.2) | < 0.001 | 60.2 (14.5) | 60.7 (16.8) | 0.81 | 64.6 (14.9) | 60.7 (14.0) | 0.02 |
| Sex (%) | ||||||||||||
| Male | 3,244 (53.1) | 185 (56.6) | 0.22 | 1,941 (52.4) | 88 (58.3) | 0.16 | 538 (56.1) | 13 (44.8) | 0.23 | 345 (51.5) | 55 (59.8) | 0.14 |
| Female | 2,867 (46.9) | 142 (43.4) | 1,761 (47.6) | 63 (41.7) | 421 (43.9) | 16 (55.2) | 325 (48.5) | 37 (40.2) | ||||
| Race (%) | ||||||||||||
| White | 4,521 (74.0) | 263 (80.4) | 0.01 | 2,733 (73.8) | 112 (74.2) | 0.31 | 686 (71.5) | 24 (82.8) | 0.25 | 540 (80.6) | 84 (91.3) | 0.04 |
| Black | 1,136 (18.6) | 39 (11.6) | 637 (17.2) | 21 (13.9) | 225 (23.5) | 3 (10.3) | 93 (13.9) | 6 (6.5) | ||||
| Other | 454 (7.4) | 25 (7.6) | 332 (9.0) | 18 (11.9) | 48 (5.0) | 2 (6.9) | 37 (5.5) | 2 (2.2) | ||||
| Detection stage (%) | ||||||||||||
| In situ | 32 (0.5) | 0 (0) | < 0.001 | 25 (0.7) | 0 (0) | 0.21 | 0 (0) | 0 (0) | 0.04 | 5 (0.7) | 0 (0) | < 0.001 |
| Localized | 1,237 (20.2) | 33 (10.1) | 617 (16.7) | 19 (12.6) | 247 (25.8) | 4 (13.8) | 211 (31.5) | 5 (5.4) | ||||
| Regional | 2,100 (34.4) | 142 (43.4) | 1,223 (33.0) | 61 (40.4) | 382 (39.8) | 16 (55.2) | 268 (40.0) | 49 (53.3) | ||||
| Distant | 2,227 (36.4) | 132 (40.4) | 1,383 (37.4) | 57 (37.7) | 314 (32.7) | 7 (24.1) | 177 (26.4) | 38 (41.3) | ||||
| Unstaged | 515 (8.4) | 20 (6.1) | 454 (12.3) | 14 (9.3) | 16 (1.7) | 2 (6.9) | 9 (1.3) | 0 (0) | ||||
| Grade differentiation (%) | ||||||||||||
| Well | 453 (7.4) | 0 (0) | < 0.001 | 266 (7.2) | 0 (0) | < 0.001 | 58 (6.0) | 0 (0) | < 0.001 | 78 (11.6) | 0 (0) | < 0.001 |
| Moderate | 2,559 (41.9) | 16 (4.9) | 1,427 (38.5) | 7 (4.6) | 519 (54.1) | 3 (10.3) | 296 (44.2) | 6 (6.5) | ||||
| Poor | 1,880 (30.8) | 229 (70.0) | 1,124 (30.4) | 103 (68.2) | 294 (30.7) | 10 (62.1) | 228 (34.0) | 71 (77.2) | ||||
| Undifferentiated | 62 (1.0) | 9 (2.8) | 28 (0.8) | 5 (3.3) | 10 (1.0) | 1 (3.4) | 14 (2.1) | 3 (3.3) | ||||
| Unknown | 1,157 (18.9) | 73 (22.3) | 857 (23.1) | 36 (23.8) | 78 (8.1) | 7 (24.1) | 54 (8.1) | 12 (13.0) | ||||
| Surgery (%) | ||||||||||||
| Yes | 3,671 (60.1) | 219 (67.0) | 0.01 | 1,579 (42.7) | 67 (44.4) | 0.68 | 865 (90.2) | 23 (79.3) | 0.06 | 632 (94.3) | 87 (94.6) | 0.93 |
| No | 2,440 (39.9) | 109 (33.0) | 2,123 (57.3) | 84 (55.6) | 94 (9.8) | 6 (20.7) | 38 (5.7) | 5 (5.4) | ||||
| Radiotherapy (%) | ||||||||||||
| Yes | 604 (9.9) | 34 (10.4) | 0.76 | 510 (13.8) | 28 (18.5) | 0.1 | 37 (3.9) | 1 (3.4) | 0.91 | 27 (4.0) | 1 (1.1) | 0.16 |
| No | 5,507 (90.1) | 293 (89.6) | 3,192 (86.2) | 123 (81.5) | 922 (96.1) | 28 (96.6) | 643 (96.0) | 91 (98.9) | ||||
| Chemotherapy (%) | ||||||||||||
| Yes | 2,452 (40.1) | 160 (48.9) | 0.002 | 1,380 (37.3) | 63 (41.7) | 0.27 | 483 (50.4) | 15 (51.7) | 0.89 | 254 (37.9) | 55 (59.8) | < 0.001 |
| No | 3,659 (59.9) | 167 (51.1) | 2,322 (62.7) | 88 (58.3) | 476 (49.6) | 14 (48.3) | 416 (62.1) | 37 (40.2) | ||||
| Incidence rate (95% CI) | 5.29 (5.18 - 5.41) | 0.27 (0.24 - 0.29) | - | 3.15 (3.07 - 3.24) | 0.14 (0.12 - 0.15) | - | 0.77 (0.73 - 0.82) | 0.02 (0.01 - 0.03) | - | 0.59 (0.55 - 0.63) | 0.07 (0.06 - 0.08) | - |
| CSS (%) (95% CI) | ||||||||||||
| 1-year | 56.2 (54.9 - 57.5) | 53.7 (48.0 - 59.1) | - | 47.5 (45.8 - 49.1) | 42.0 (33.9 - 49.8) | - | 76.9 (74.0 - 79.6) | 58.7 (35.5 - 76.1) | - | 72.7 (69.0 - 76.0) | 72.3 (61.8 - 80.4) | - |
| 2-year | 42.3 (41.0 - 43.7) | 30.1 (24.7 - 35.6) | 33.6 (31.9 - 35.3) | 22.5 (15.7 - 30.0) | 62.8 (59.5 - 66.0) | 24.5 (9.0 - 44.0) | 59.9 (55.8 - 63.8) | 38.6 (27.9 - 49.2) | ||||
| 5-year | 28.2 (26.7 - 29.3) | 16.4 (12.0 - 21.3) | 22.7 (19.5 - 22.6) | 17.8 (11.6 - 25.1) | 41.5 (37.9 - 45.1) | 24.5 (9.0 - 44.0) | 46.3 (42.0 - 50.5) | 7.9 (3.0 - 15.8) | ||||
| 10-year | 23.8 (22.5 - 25.1) | 13.8 (9.7 - 18.8) | 18.8 (16.0 - 19.1) | 16.2 (10.0 - 23.6) | 40.9 (32.6 - 40.0) | 16.3 (3.6 - 37.2) | 41.7 (37.1 - 46.2) | 5.9 (1.8 - 13.6) | ||||
| Median (Months) | 16.5 | 14.2 | 10.9 | 5.7 | 40.9 | 13.3 | 39.3 | 20.0 | ||||
| Location | Univariate (95% CI) | Multivariable (95% CI) |
|---|---|---|
| Multivariable hazard ratios were corrected for age, sex, race, detection stage, grade differentiation, surgery, radiotherapy, and chemotherapy. CI: confidence interval. | ||
| Small bowel | 1.35 (1.18 - 1.54) | 1.23 (1.06 - 1.41) |
| Duodenum | 1.25 (1.02 - 1.52) | 1.19 (0.97 - 1.46) |
| Jejunum | 1.94 (1.24 - 3.04) | 1.87 (1.19 - 2.95) |
| Ileum | 2.23 (1.71 - 2.90) | 1.25 (0.93 - 1.69) |