Figures
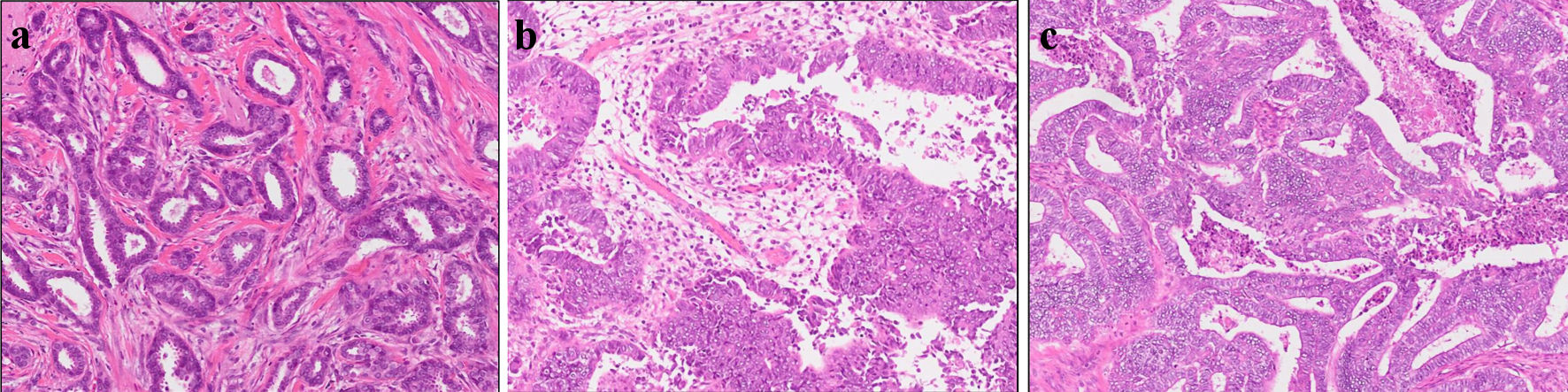
Figure 1. Histopathological results. Pathological analysis of surgical tissue from (a) breast, (b) uterus, and (c) abdominal tumor.
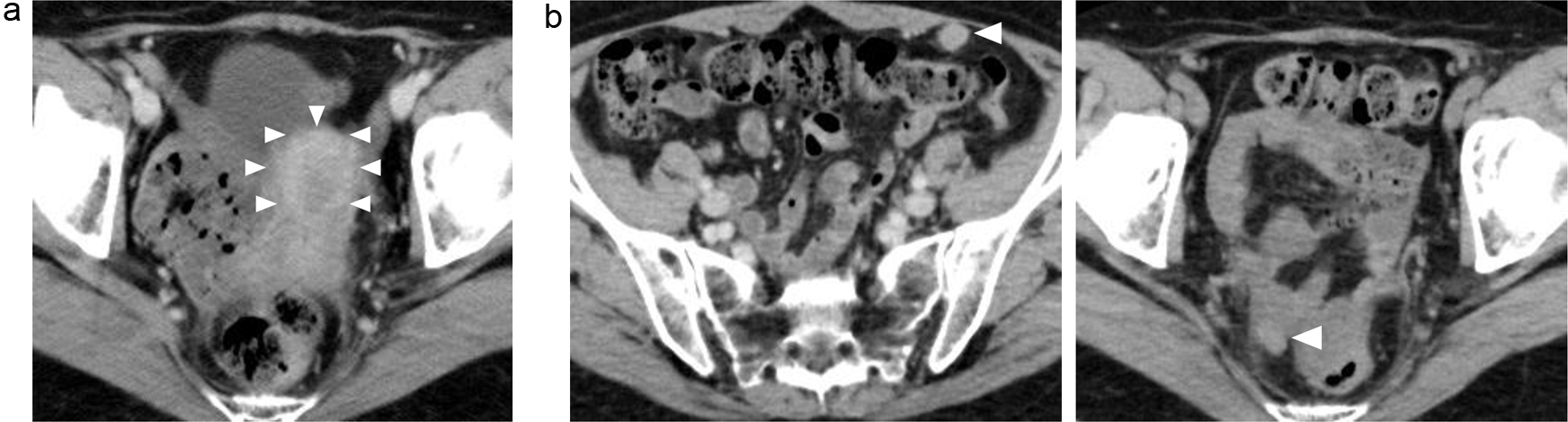
Figure 2. (a, b) Abdominal CT images revealing the multiple abdominal mass (arrows). CT: computed tomography.
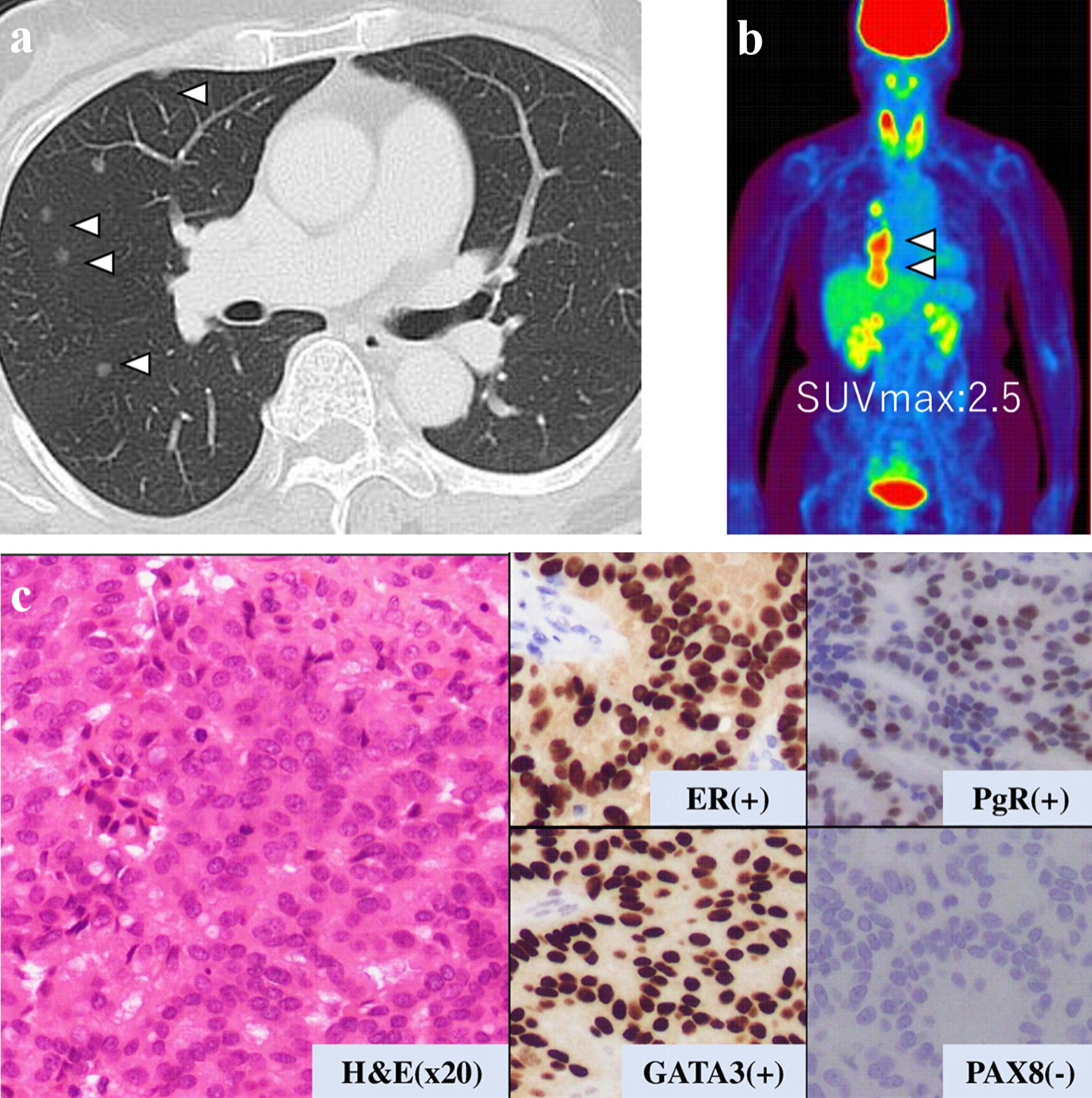
Figure 3. Examination results for mass in her thoracic cavity by (a) CT, (b) PET-CT (SUVmax = 2.5), and (c) pathological analysis. ER: estrogen receptor; PgR: progesterone receptor; H&E: hematoxylin and eosin stain; CT: computed tomography; PET-CT: positron emission tomography-computed tomography; SUVmax: maximum standardized uptake value.
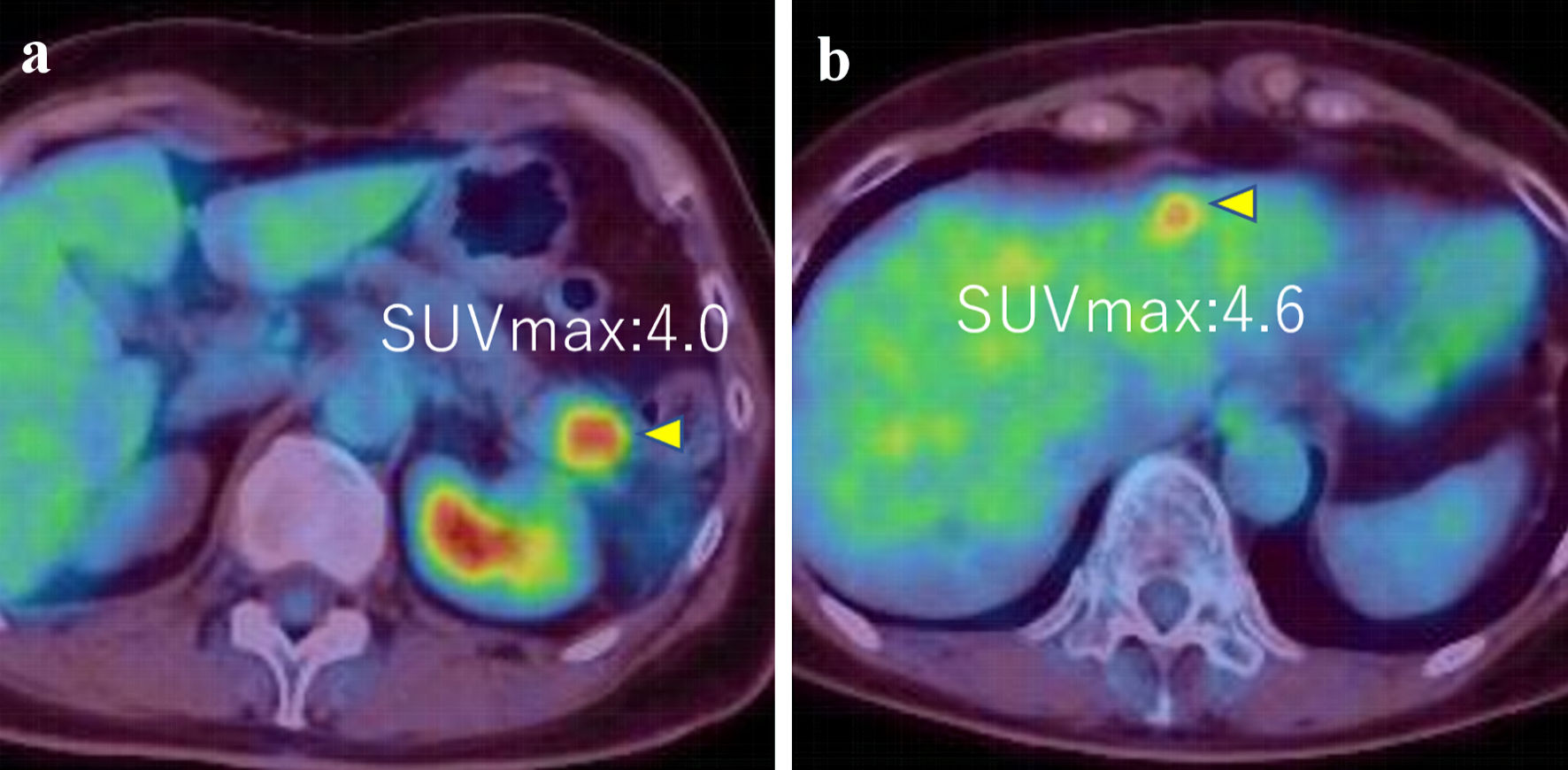
Figure 4. (a, b) PET-CT images showed tumors in pancreas and liver (SUVmax = 4.0 and 4.6, respectively) (arrows). PET-CT: positron emission tomography-computed tomography; SUVmax: maximum standardized uptake value.
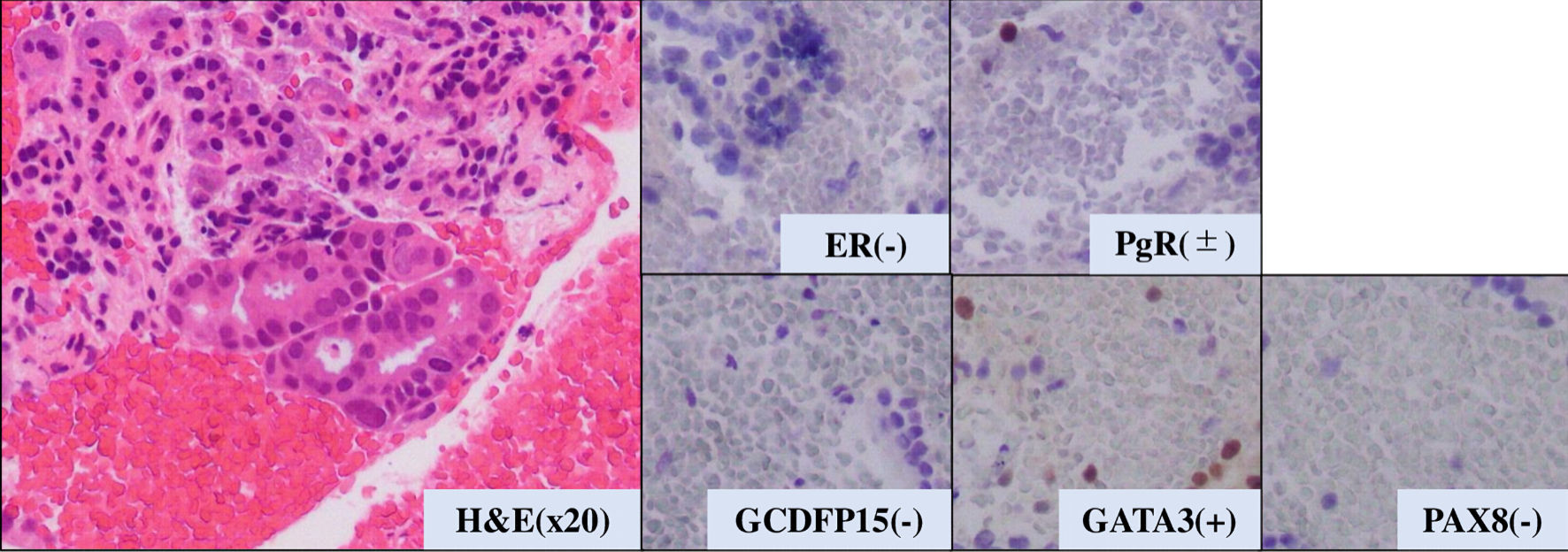
Figure 5. Pathological analysis of pancreatic tumor tissue. H&E: hematoxylin and eosin stain. ER: estrogen receptor; PgR: progesterone receptor; H&E: hematoxylin and eosin stain; GATA-3: GATA binding protein 3; PAX8: paired box 8; GCDFP15: gross cystic disease fluid protein 15.
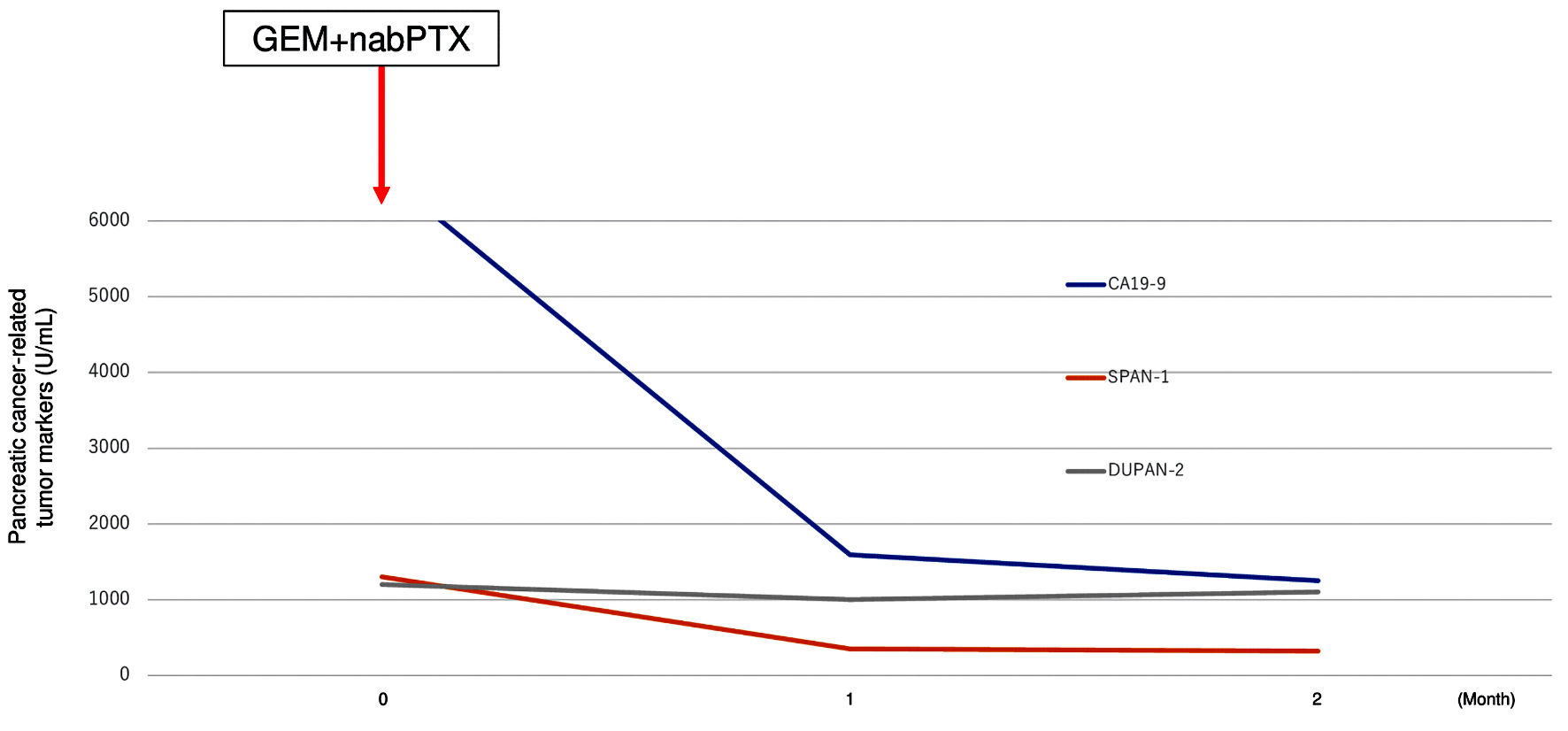
Figure 6. Transition graph of tumor markers (CA19-9, SPAN-1, and DUPAN-2). GEM+nabPTX: gemcitabine with nab-paclitaxel; DUPAN-2: Duke pancreatic monoclonal antigen type 2; SPAN-1: s-pancreas antigen-1; CA19-9: carbohydrate antigen 19-9.
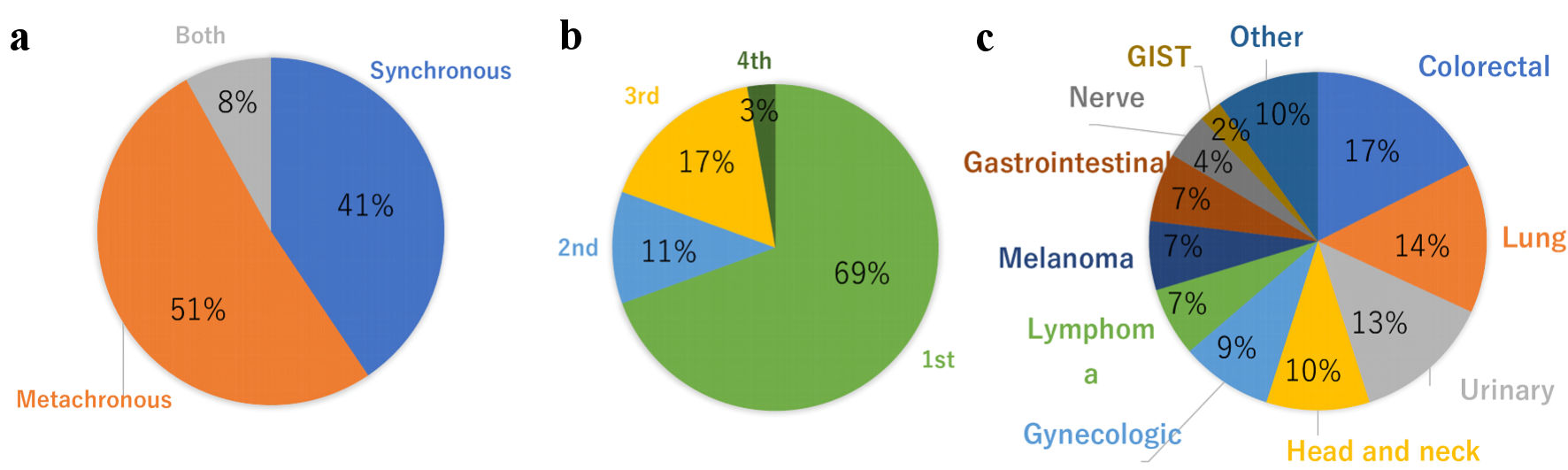
Figure 7. Distribution of MPMNs with breast cancer patients. (a) Synchronous vs. metachronous. (b) Breast cancer onset timing. (c) Comorbid cancer. MPMNs: multiple primary malignant neoplasms; GIST: gastrointestinal stromal tumor.







