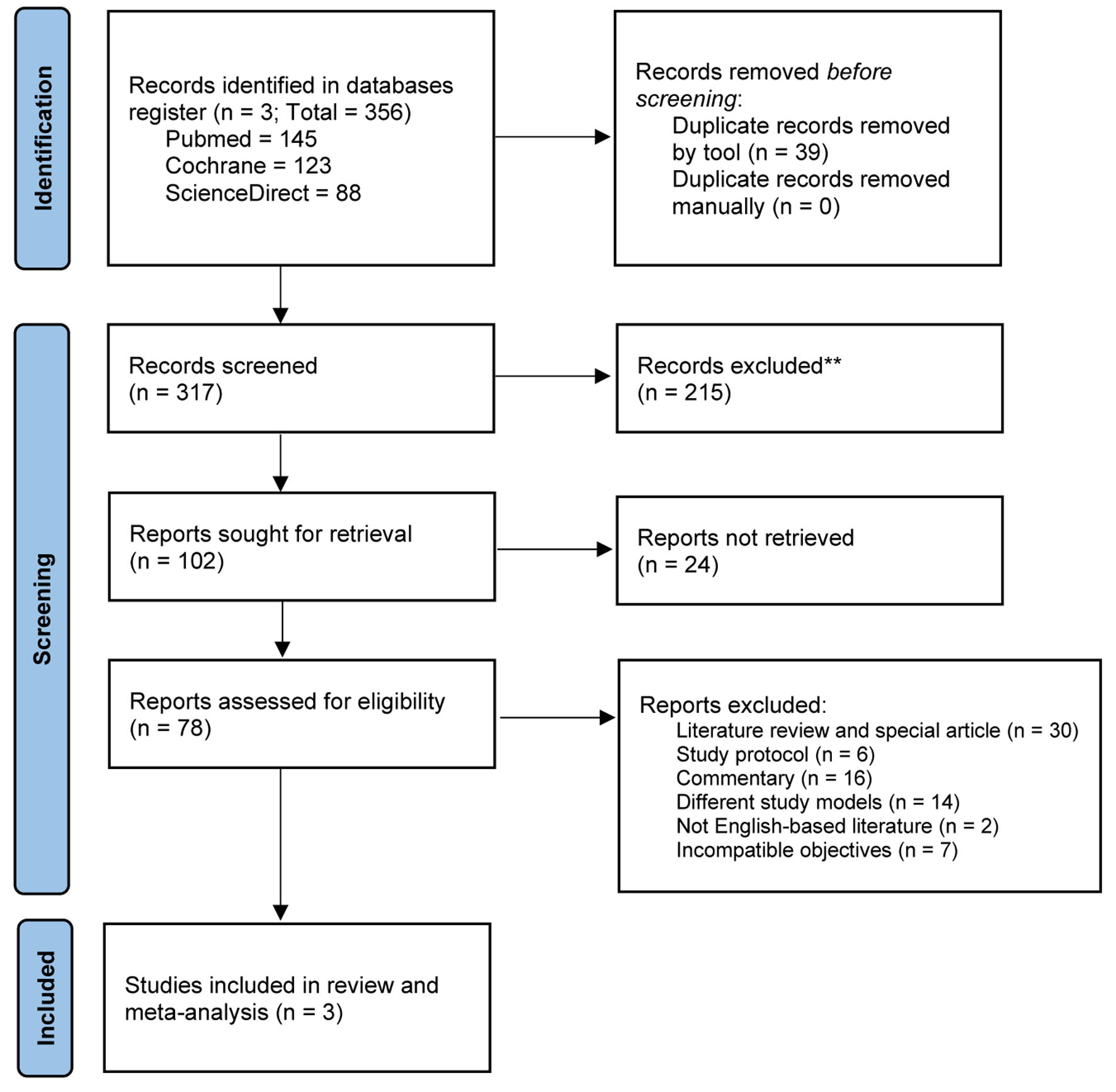
Figure 1. PRISMA reporting diagram to identify eligible trials for review.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 518-528
Addition of Olaparib to the New Hormonal Agent Regimen for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis
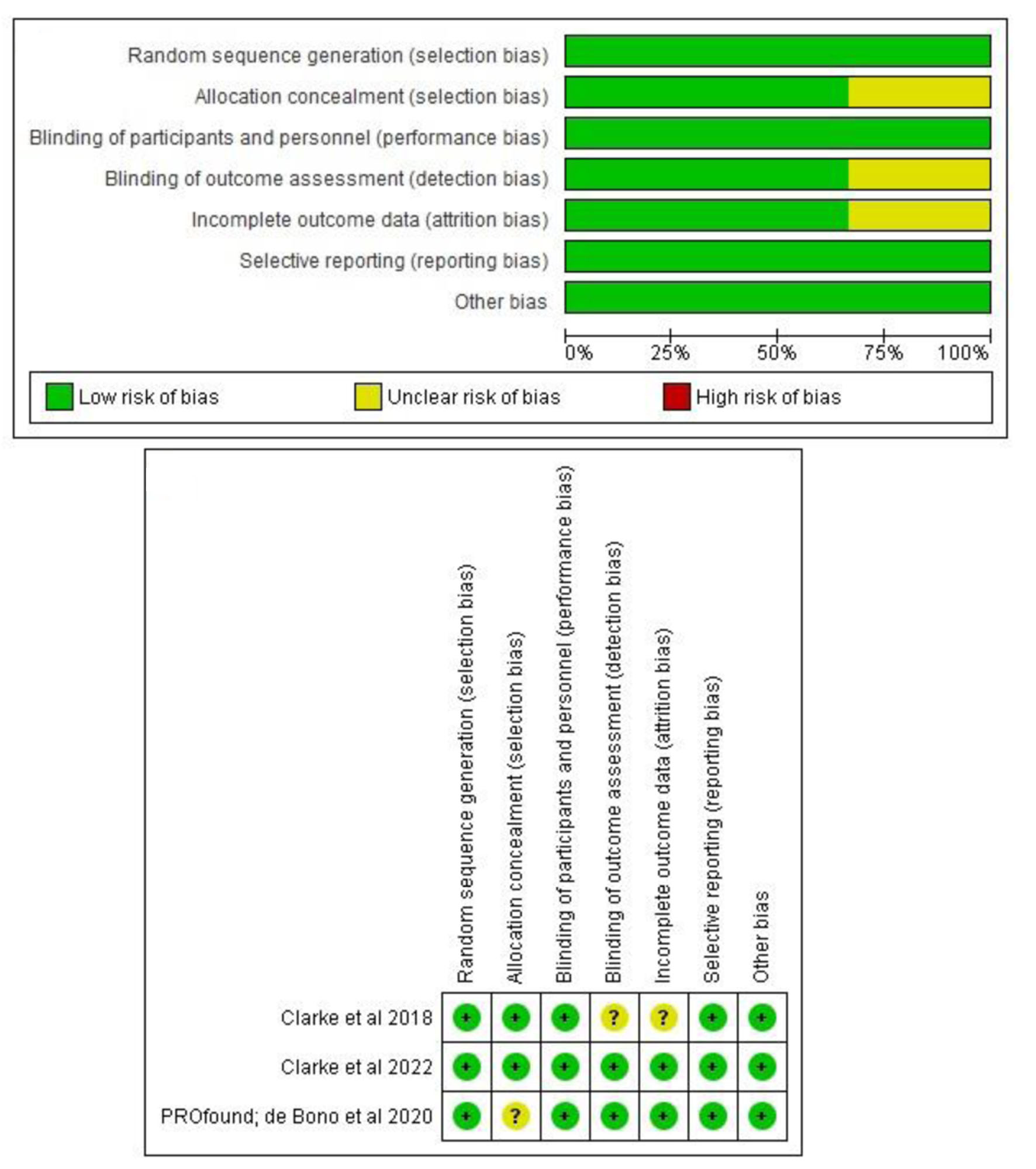
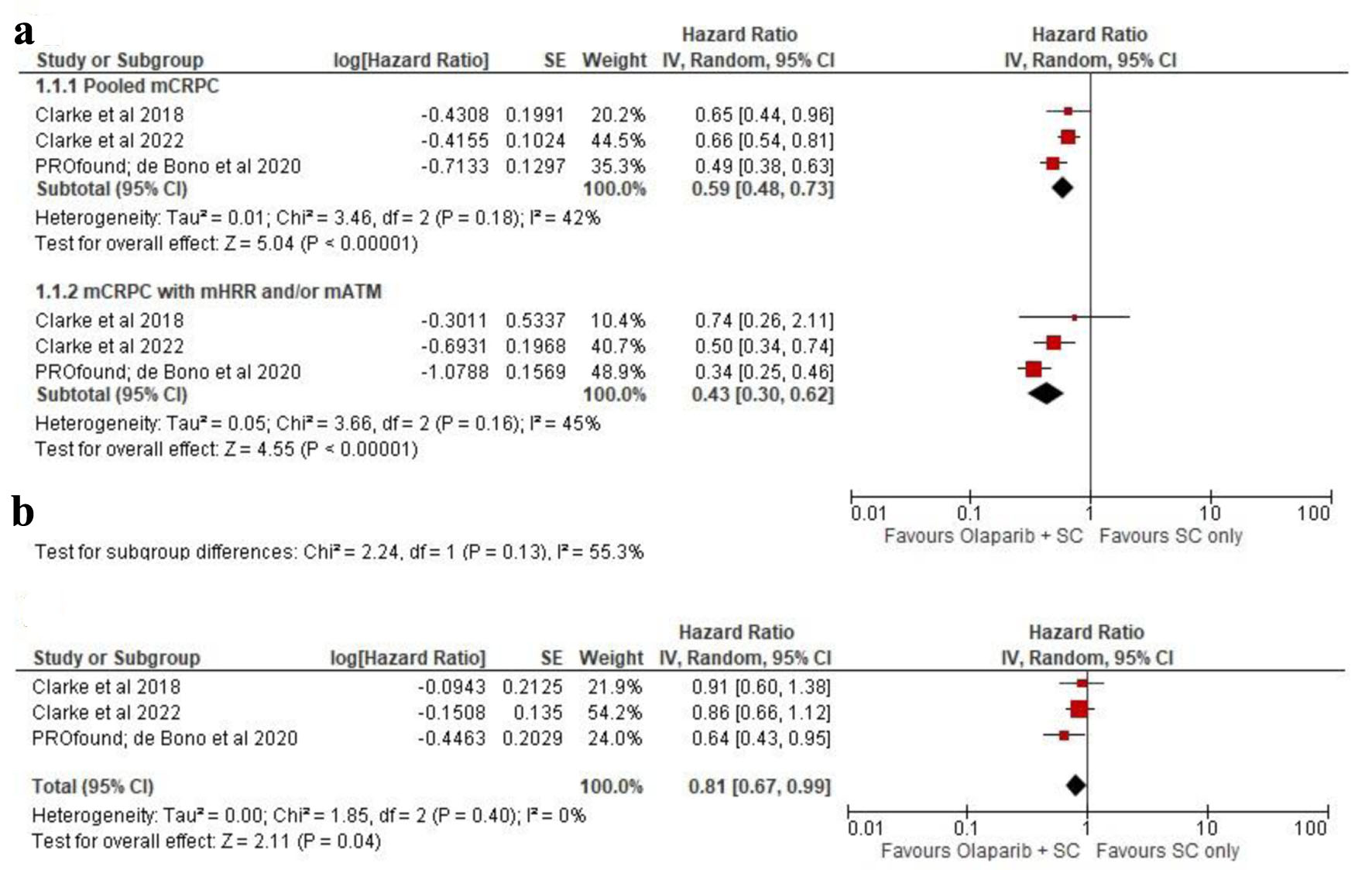
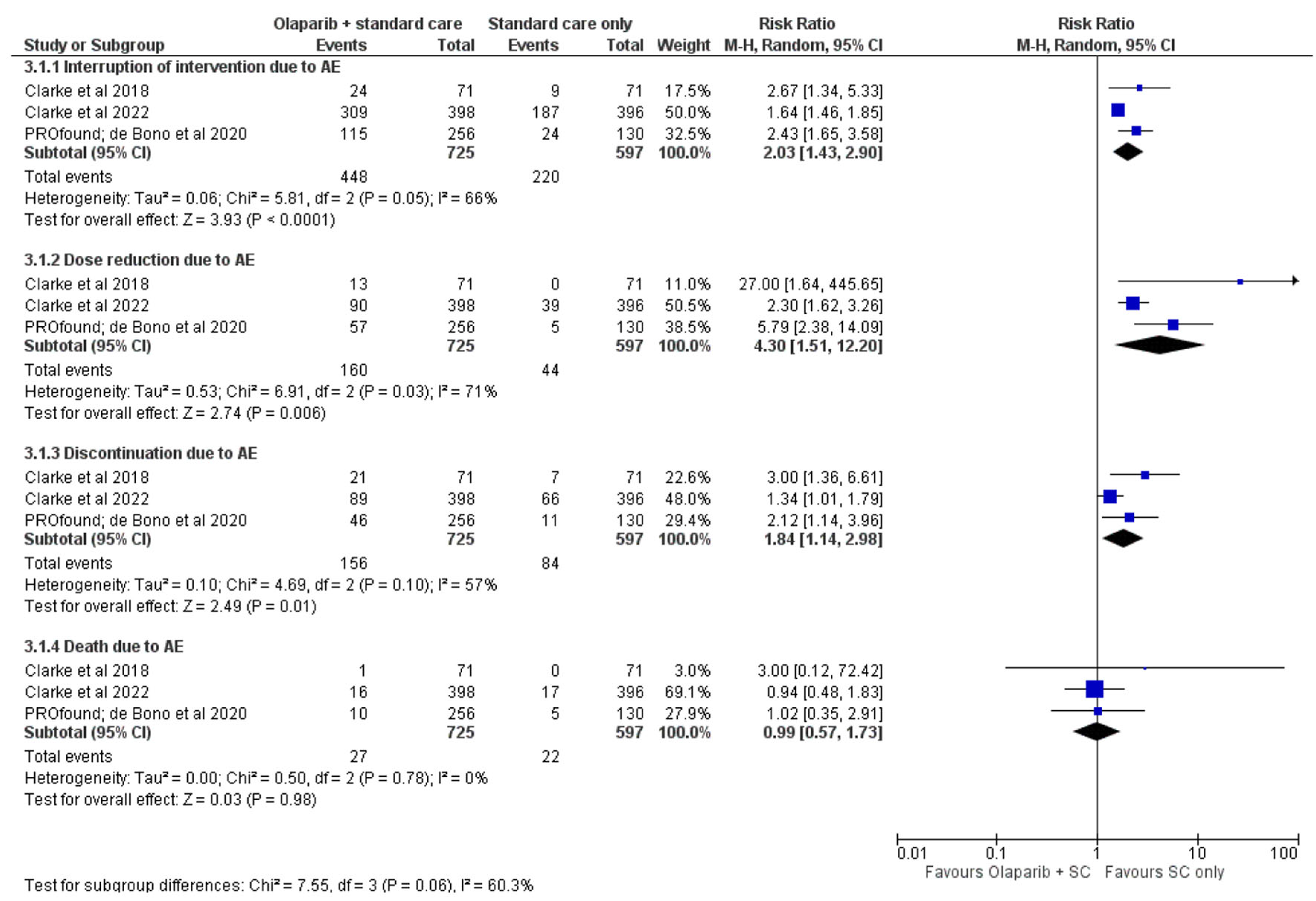
Figures

Tables
| Study and design | Criteria of eligibility | Treatment settings | Intervention (olaparib-regimen) | Comparison | Maximum trial follow-up (months) | Outcomes |
|---|---|---|---|---|---|---|
| RCT: randomized controlled trial; mCRPC: metastatic castration-resistant prostate cancer; ADT: androgen deprivation therapy; CT: computed tomography; MRI: magnetic resonance imaging; mHSPC: metastatic hormone-sensitive prostate cancer. | ||||||
| Clarke 2018 (open-label RCT phase II) | Males ≥ 18 years old or older; Histologically or cytologically confirmed mCRPC | Patients with previous taxanes treatment were allowed | Olaparib (300 mg) PO bd + abiraterone (1,000 mg) PO qd + prednisone/prednisolone (5 mg) PO bd | Placebo + abiraterone (1,000 mg) PO qd + prednisone/prednisolone (5 mg) PO bd | 30 | Progression-free survival, overall survival, adverse events |
| Clarke 2022 (RCT phase III) | Male ≥ 18 years old or older (≥ 19 years in South Korea centers); Histologically or cytologically confirmed CRPC; At least one documented metastatic lesion on a bone scan or CT or MRI scan | First-line treatment after mCRPC confirmation (except for ADT); Prior docetaxel during neoadjuvant/adjuvant treatment for mHSPC | Olaparib (300 mg) PO bd + abiraterone (1,000 mg) PO qd + prednisone/prednisolone (5 mg) PO bd | Placebo + abiraterone (1,000 mg) PO qd + prednisone/prednisolone (5 mg) PO bd | 31 | Progression-free survival, overall survival, adverse events |
| PROfound: De Bono 2020 (open-label RCT phase III) | Male ≥ 18 years old or older; Histologically or cytologically confirmed mCRPC | Administered with docetaxel as well for treating the current mCRPC | Olaparib (300 mg) PO bd + abiraterone (1,000 mg) PO qd + prednisone/prednisolone (5 mg) PO bd | Placebo + abiraterone (1,000 mg) PO qd + prednisone/prednisolone (5 mg) PO bd | 31 | Progression-free survival, overall survival, adverse events |
| Study/arm (n) | Age (years) | ECOG performance status ≥ 1 (%) | Metastasis status and number of bone metastases at the baseline (%) | Prior treatment (%) | PSA concentration at the baseline (µg/L) | Confirmed HRR mutation and BRCA status (%) | |
|---|---|---|---|---|---|---|---|
| ECOG: Eastern Cooperative Oncology Group; PSA: prostate-specific antigen; HRR: homologous recombinant repair; NHA: new hormonal agent. | |||||||
| Clarke 2018 | Olaparib (71) | 70 (65 - 75) | 52.1 | Bone disease only (46.5); Soft-tissue disease only (11.3); Bone and soft-tissue disease (42.3); Number of participants with bone metastasis sites > 1 (88.7) | Docetaxel (100.0); Cabazitaxel (14.1) | 86.0 (23 - 194) | HRR mutation (15.5) |
| Control (71) | 67 (62 - 74) | 43.6 | Bone disease only (46.5); Soft-tissue disease only (15.5); Bone and soft-tissue disease (38.0); Number of participants with bone metastasis sites > 1 (85.9) | Docetaxel (100.0); Cabazitaxel (12.7); Abiraterone (1.4) | 47.0 (21 - 199) | HRR mutation (14.1) | |
| Clarke 2022 | Olaparib (399) | 69 (43 - 91) | 28.1 | Bone-related (87.5); Distant lymph nodes (33.3); Locoregional lymph nodes (20.6); Prostate and adjacent structure (11.8); Respiratory (including lung) (10.6); Liver (3.8) | Docetaxel (46.9); NHA (0.3) | 17.9 (6.1 - 67.0) | HRR mutation (27.8); BRCA1 (2.3); BRCA2 (9.5) |
| Control (397) | 70 (46 - 88) | 31.2 | Bone-related (85.4); Distant lymph nodes (30.0); Locoregional lymph nodes (22.4); Prostate and adjacent structure (11.6); Respiratory (including lung) (10.6); Liver (4.5) | Docetaxel (46.9); NHA (0.3) | 16.8 (6.3 - 53.3) | HRR mutation (27.8); BRCA1 (2.3); BRCA2 (9.5) | |
| De Bono 2020 | Olaparib (256) | 69 (47 - 91) | 48.8 | Bone disease only (33.6); Lung or liver (26.6); Other (34.4) | NHA (100.0); Taxanes (66.4) | 68.2 (24.1 - 294.4) | BRCA1 (3.1); BRCA2 (31.6) |
| Control (131) | 69 (49 - 87) | 57.3 | Bone disease only (29.0); Lung or liver (33.6); Other (31.3) | NHA (100.0); Taxanes (66.4) | 106.5 (37.2 - 326.6) | BRCA1 (3.8); BRCA2 (35.9) | |
| Adverse effects | All grades, % (n/total) | Grade ≥ 3, % (n/total) | ||||||
|---|---|---|---|---|---|---|---|---|
| Olaparib plus SC (NHA + CtS) | SC (NHA + CtS) only | RR (95% CI) | P value | Olaparib plus SC (NHA + CtS) | SC (NHA + CtS) only | RR (95% CI) | P value | |
| *Statistically significant based on the respective analysis model. CI: confidence interval; CtS: corticosteroid; NHA: new hormonal agent; RR: risk ratio; UTI: urinary tract infection. | ||||||||
| Hematological | ||||||||
| Anemia | 44.7 (324/725) | 14.4 (86/597) | 3.16 (2.06 - 4.84) | < 0.05* | 17.9 (130/725) | 3.5 (21/597) | 4.64 (2.96 - 7.28) | < 0.05* |
| Non-hematological | ||||||||
| Nausea | 33.8 (245/725) | 15.1 (90/597) | 2.13 (1.71 - 2.64) | < 0.05* | 0.7 (5/725) | 0.5(3/597) | 1.05 (0.23 - 4.92) | > 0.05* |
| Decreased appetite | 20.3 (147/725) | 8.5 (51/597) | 2.05 (1.53 - 2.76) | < 0.05* | 1.0 (7/725) | 0.2(1/597) | 2.95 (0.50 - 17.57) | > 0.05* |
| Diarrhea | 18.5 (134/725) | 8.9 (53/597) | 2.02 (1.48 - 2.74) | < 0.05* | 0.7 (5/725) | 0.3(2/597) | 1.67 (0.35 - 8.08) | > 0.05* |
| Vomiting | 15.7 (114/725) | 10.2 (61/597) | 1.49 (1.11 - 2.00) | < 0.05* | 3.4 (25/725) | 0.5 (3/597) | 5.92 (1.77 - 19.82) | < 0.05* |
| Constipation | 18.2 (132/725) | 13.7 (82/597) | 1.33 (1.01 - 1.74) | < 0.05* | 0.0 (0/725) | 0.2 (1/597) | 0.33 (0.01 - 8.12) | > 0.05* |
| Fatigue | 37.0 (268/725) | 27.3 (163/597) | 1.31 (1.12 - 1.55) | < 0.05* | 2.3 (17/725) | 2.5 (15/597) | 0.82 (0.38 - 1.78) | > 0.05* |
| UTI | 9.4 (68/725) | 8.2 (49/597) | 1.15 (0.81 - 1.62) | > 0.05* | 1.8 (13/725) | 1.8 (11/597) | 0.92 (0.43 - 1.99) | > 0.05* |
| Peripheral edema | 11.9 (86/725) | 10.1 (63/597) | 1.14 (0.83 - 1.56) | > 0.05* | 0.0 (0/725) | 0.2 (1/597) | 0.33 (0.01, 8.12) | > 0.05* |
| Back pain | 16.7 (121/725) | 17.1 (102/597) | 1.02 (0.80 - 1.30) | > 0.05* | 0.8 (6/725) | 1.0 (7/725) | 0.69 (0.23 - 2.06) | > 0.05* |
| Arthralgia | 11.4 (83/725) | 14.7 (88/597) | 0.85 (0.57 - 1.28) | > 0.05* | 0.1 (1/725) | 0.5 (3/597) | 0.45 (0.07 - 2.76) | > 0.05* |