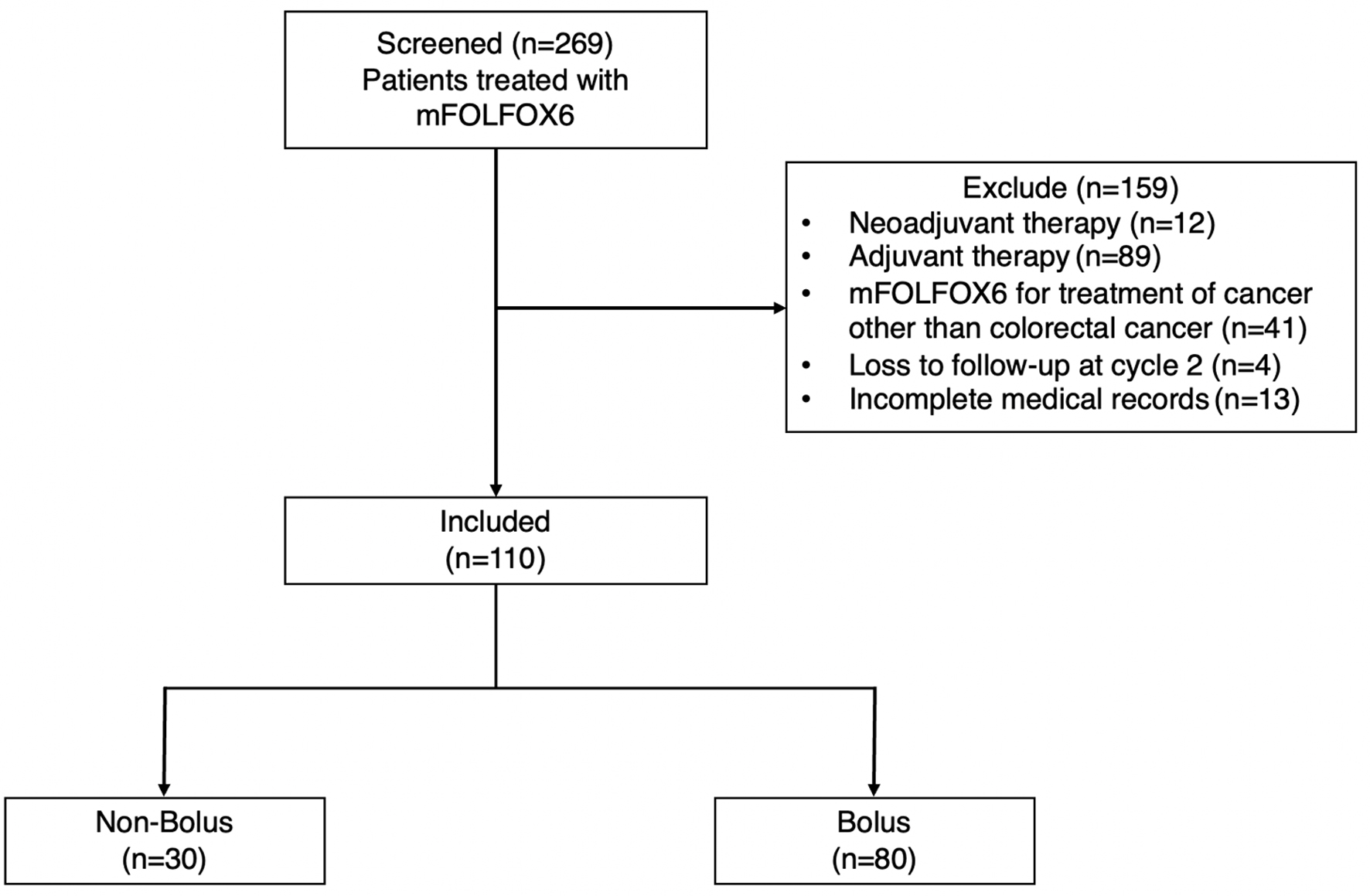
Figure 1. Flow diagram of patient selection.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 5, October 2023, pages 392-400
The Impact of Omitting 5-FU Bolus From mFOLFOX6 Chemotherapy Regimen on Hematological Adverse Events Among Patients With Metastatic Colorectal Cancer
Figures

Tables
| Baseline characteristics | Non-bolus (N = 30) | Bolus (N = 80) | P-value |
|---|---|---|---|
| aOther comorbidities: skin diseases, eye disorders, psychiatric disorders, chronic kidney disease, neurological disorders, pulmonary disease. bOther sites of metastasis: spleen, gastric, peritoneal, ovary, lymph node, Mx. cTargeted therapy: bevacizumab, cetuximab, panitumumab. *Significantly different at P-value < 0.05. BSA: body surface area; BMI: body mass index; 5-FU: 5-fluorouracil; LV: leucovorin; SD: standard deviation. | |||
| Characteristics | |||
| Age (years), mean ± SD | 72.4 ± 11.7 | 66.1 ± 10.7 | 0.0085* |
| Male, n (%) | 15 (50.0) | 43 (53.8) | 0.8309 |
| Weight (kg), mean ± SD | 52.6 ± 8.6 | 59.3 ± 11.7 | 0.1890 |
| BSA (m2), mean ± SD | 1.5 ± 0.2 | 1.6 ± 0.2 | 0.0214* |
| BMI (kg/m2), mean ± SD | 21.1 ± 3.2 | 22.6 ± 3.9 | 0.0627 |
| Comorbidities, n (%) | |||
| Cardiovascular disease | 16 (53.3) | 58 (72.5) | 0.0696 |
| Endocrine disorders | 7 (23.3) | 24 (30.0) | 0.6353 |
| Gynecological disease | 2 (6.7) | 3 (3.8) | 0.6123 |
| Othersa | 4 (13.3) | 10 (12.5) | 1.0000 |
| Medications, n (%) | |||
| Cardiovascular medications | 14 (46.7) | 58 (72.5) | 0.0005* |
| Endocrine medications | 5 (16.7) | 17 (21.3) | 0.7899 |
| Genitourinary medications | 2 (6.7) | 5 (6.3) | 1.0000 |
| Neuropsychiatric medications | 4 (13.3) | 6 (7.5) | 0.4561 |
| Respiratory medications | 2 (6.7) | 4 (5.0) | 0.6633 |
| Gastrointestinal medications | 3 (10.0) | 5 (6.3) | 0.6809 |
| Elemental nutrition | 3 (10.0) | 8 (10.0) | 1.0000 |
| Number of metastatic sites, n (%) | |||
| Single site | 17 (56.7) | 58 (72.5) | 0.1669 |
| Multiple sites | 13 (43.3) | 22 (27.5) | |
| Site of metastasis, n (%) | |||
| Lung | 12 (40.0) | 26 (32.5) | 0.5038 |
| Liver | 18 (60.0) | 44 (55.0) | 0.6719 |
| Bone and soft tissue | 2 (6.7) | 5 (6.3) | 1.0000 |
| Otherb | 10 (33.3) | 40 (50.0) | 0.1365 |
| Lines of mFOLFOX6 regimen, n (%) | |||
| First line | 27 (90.0) | 61 (76.3) | 0.1790 |
| Second line | 3 (10.0) | 19 (23.8) | |
| Previous exposure to chemotherapy, n (%) | |||
| 5-FU/LV based regimen/capecitabine | 2 (6.7) | 12 (15.0) | 0.3429 |
| mFOLFIRI ± targeted therapyc/IFL ± targeted therapyc | 0 (0.0) | 4 (5) | 0.5731 |
| mFOLFOX/XELOX | 1 (3.3) | 6 (7.5) | 0.6741 |
| Dosing and regimen | Non-bolus (N = 30) | Bolus (N = 80) | P-value |
|---|---|---|---|
| aTargeted therapy: bevacizumab, cetuximab, panitumumab. *Significantly different at P-value < 0.05. N/A: not available; 5-FU: 5-fluorouracil; SD: standard deviation. | |||
| Duration between first and second cycles (days), mean ± SD | 15.6 ± 3.9 | 16.9 ± 5.9 | 0.2664 |
| Treatment regimen, n (%) | |||
| mFOLFOX6 + targeted therapya | 4 (13.3) | 11 (13.8) | 1.0000 |
| mFOLFOX6 | 26 (86.7) | 69 (86.3) | 1.0000 |
| Treatment dose (mg/m2), mean ± SD | |||
| 5-FU bolus | - | 637.7 ± 75.2 | N/A |
| 5-FU infusion | 1,769.7 ± 193.0 | 1,848.7 ± 352.8 | 0.2483 |
| Leucovorin | 295.5 ± 29.6 | 316.4 ± 43.8 | 0.0176* |
| Oxaliplatin | 108.8 ± 17.4 | 134.7 ± 25.7 | < 0.0001* |
| First-line treatment dose (mg/m2), mean ± SD | Non-bolus (N = 27) | Bolus (N = 61) | |
| 5-FU bolus | - | 637.6 ± 71.4 | N/A |
| 5-FU infusion | 1,778.9 ± 195.4 | 1,864.5 ± 283.5 | 0.1582 |
| Leucovorin | 295.5 ± 29.6 | 315.8 ± 43.9 | 0.0313* |
| Oxaliplatin | 110.6 ± 17.2 | 135.0 ± 27.5 | < 0.0001* |
| Adverse events outcome | Non-bolus (N = 30) | Bolus (N = 80) | OR (95% CI), P-value |
|---|---|---|---|
| *Significantly different at P-value < 0.05. ANC: absolute neutrophil count; CI: confidence interval; Hb: hemoglobin; N/A: not available; OR: odds ratio; Plt: platelet. | |||
| Hematological adverse events, n (%) | |||
| Any grade | |||
| Neutropenia | 0 (0.0) | 5 (6.3) | 4.44 (0.24, 82.90), 0.3023 |
| Anemia | 6 (20.0) | 22 (27.5) | 1.52 (0.55, 4.21), 0.4722 |
| Thrombocytopenia | 0 (0.0) | 1 (1.3) | 1.15 (0.04, 29.05), 1.0000 |
| Grade 3/4 | |||
| Neutropenia | 0 (0.0) | 1 (1.3) | 1.15 (0.04, 29.05), 1.0000 |
| Anemia | 1 (3.3) | 3 (3.8) | 1.13 (0.11, 11.31), 1.0000 |
| % Reduction, mean | Mean difference, P-value | ||
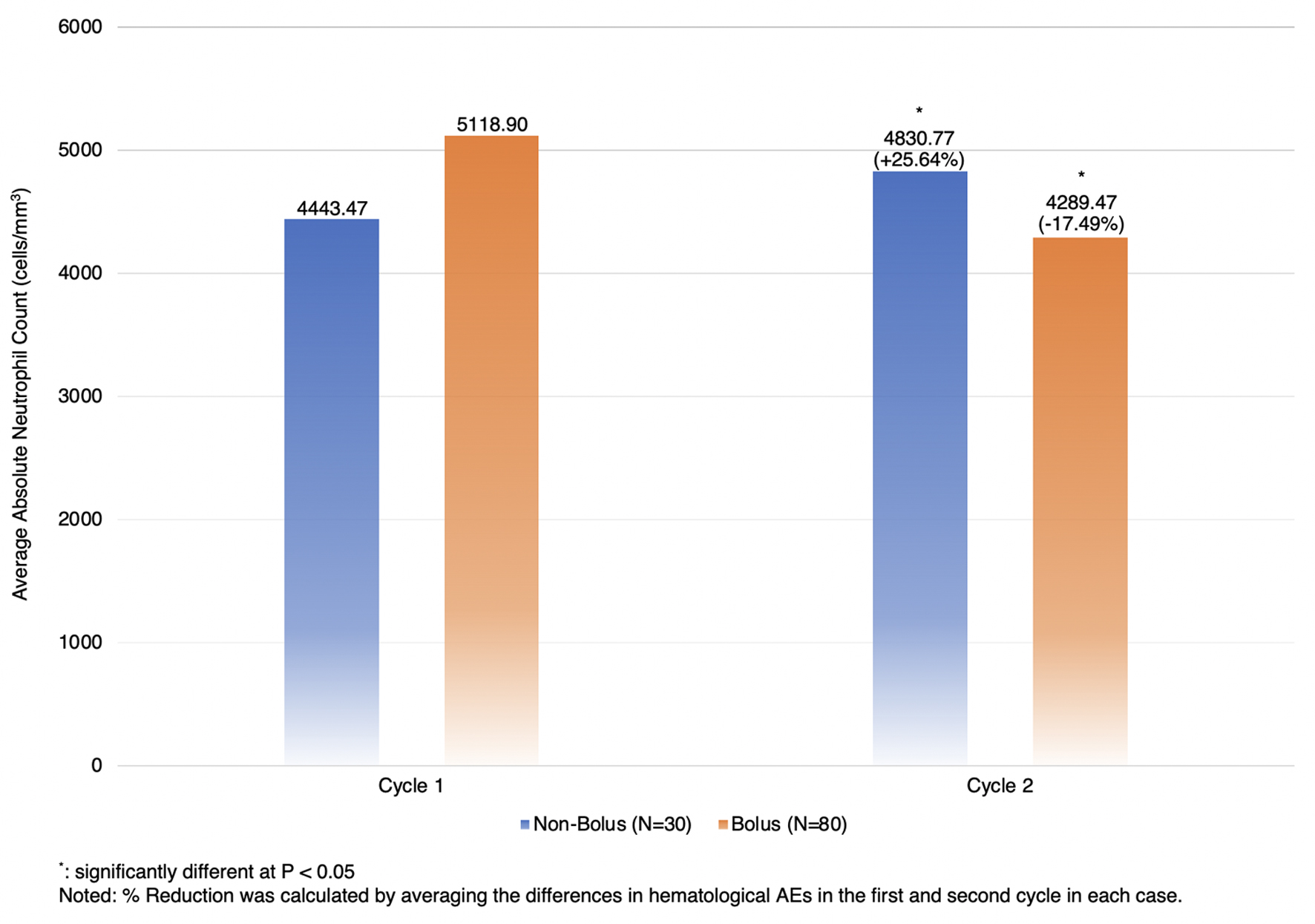
| ANC | +25.64%, ± 79.99 | -17.49%, ± 38.05 | 43.13 (20.74, 65.51), 0.0002* |
| Hb | +0.31%, ± 12.77 | -0.74%, ± 15.40 | 1.05 (-7.31, 5.21), 0.7402 |
| Plt | -9.98%, ± 18.96 | -14.48%, ± 25.61 | 4.763 (-14.97, 5.44), 0.3567 |
| Cycle two growth factor added | 0 (0.0) | 1 (1.3) | 1.15 (0.04, 29.05), 1.000 |
| Other adverse event, n (%) | OR (95% CI), P-value | ||
| Any grade | |||
| Mucositis | 0 (0.0) | 7 (8.8) | 6.22 (0.34, 112.50), 0.1863 |
| Nausea and vomiting | 0 (0.0) | 3 (3.8) | 2.75 (0.14, 54.97), 0.5607 |
| Diarrhea | 0 (0.0) | 0 (0.0) | N/A |
| Adverse events | Non-bolus (N=27) | Bolus (N = 61) | OR (95% CI), P-value |
|---|---|---|---|
| *Significantly different at P-value < 0.05. ANC: absolute neutrophil count; CI: confidence interval; Hb: hemoglobin; N/A: not available; OR: odds ratio; Plt: platelet. | |||
| First-line hematological adverse events, n (%) | |||
| Any grade | |||
| Neutropenia | 0 (0.0) | 5 (8.2) | 5.35 (0.28, 100.40), 0.3179 |
| Anemia | 6 (22.2) | 20 (32.8) | 1.71 (0.59, 4.89), 0.4480 |
| Grade 3/4 | |||
| Neutropenia | 0 (0.0) | 1 (1.6) | 1.36 (0.05, 34.58), 1.0000 |
| Anemia | 1 (3.7) | 3 (4.9) | 1.34 (0.13, 13.56), 1.0000 |
| % Reduction, mean | Mean difference, P-value | ||
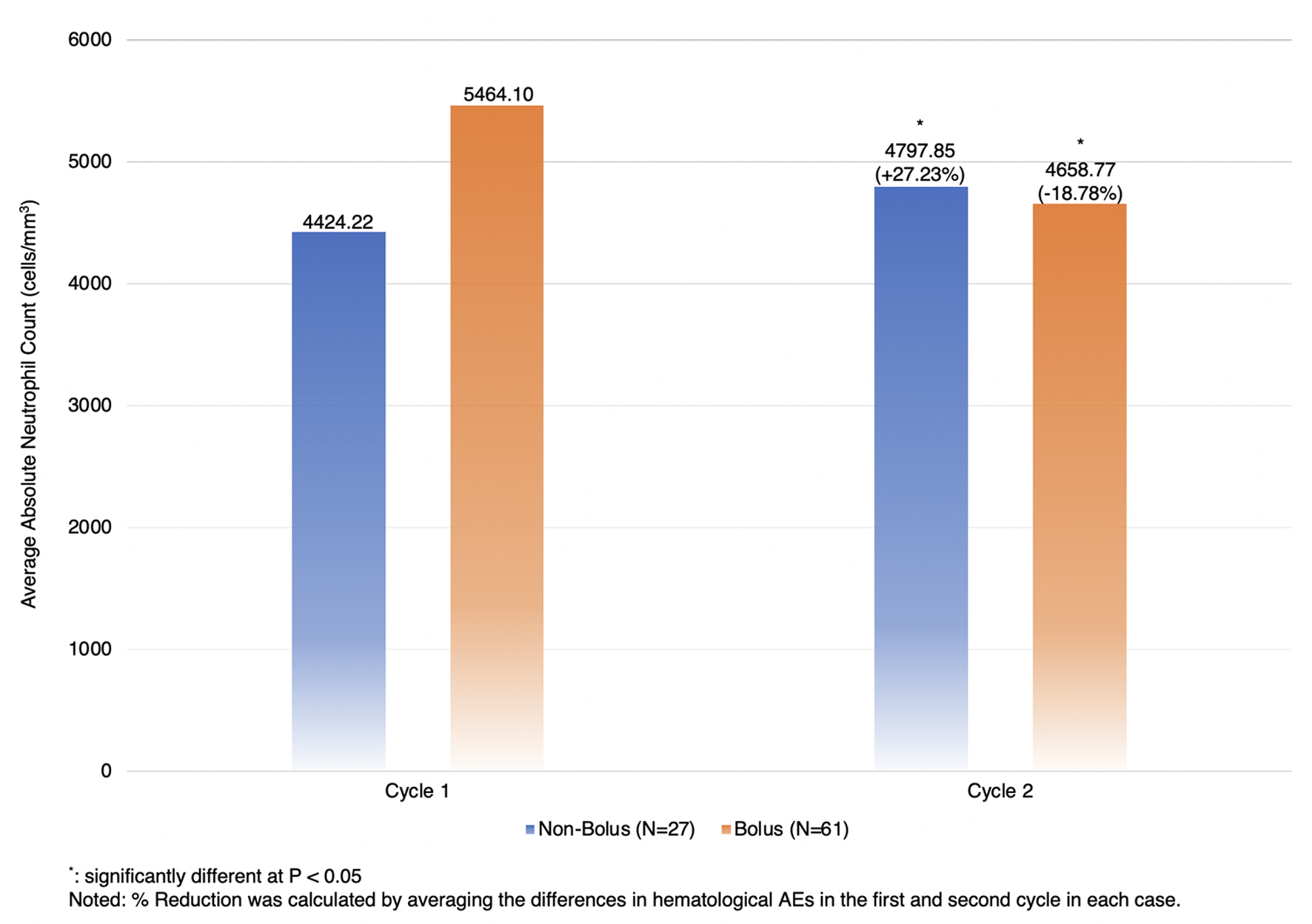
| ANC | +27.23%, ± 84.29 | -18.78%, ± 38.77 | 46.01 (19.99, 72.03), 0.0007* |
| Hb | -0.36%, ± 13.27 | -0.55%, ± 17.12 | 0.19 (-7.58, 7.20), 0.9593 |
| Plt | -10.38%, ± 19.77 | -14.71%, ± 23.88 | 4.33 (-14.79, 6.126), 0.4119 |
| First-line other adverse events, n (%) | |||
| Any grade | |||
| Mucositis | 0 (0.0) | 3 (4.9) | 3.29 (0.16, 65.99), 0.5497 |
| Nausea and vomiting | 0 (0.0) | 2 (3.3) | 2.31 (0.11, 49.81), 1.0000 |
| Diarrhea | 0 (0.0) | 0 (0.0) | N/A |