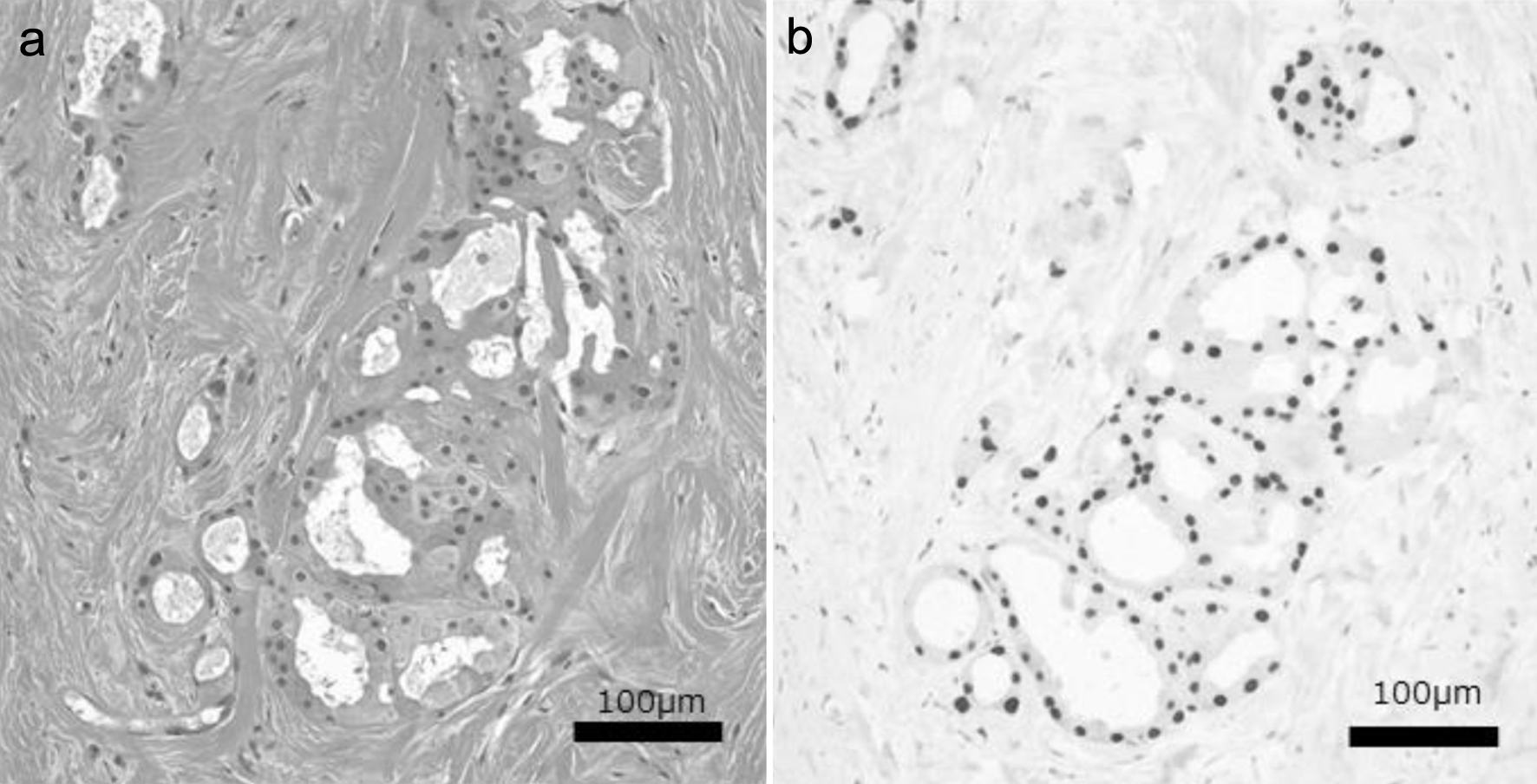
Figure 1. Hematoxylin and eosin (a) and nuclear AR (b) staining images of a representative TN-PAC case. TN-PAC: triple-negative pure apocrine carcinoma; AR: androgen receptor.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 551-557
Clinicopathological Characteristics and Prognosis of Triple-Negative Apocrine Carcinoma: A Case-Control Study
Figures

Table
| All cohort (N = 53) | TN-PAC (N = 24) | Other-AC (N = 29) | P-value | |
|---|---|---|---|---|
| AC: apocrine carcinoma; Ax: axillary lymph node dissection; BCS: breast conserving surgery; HER2: human epidermal growth factor receptor 2; NA: not assessed; PAC: pure apocrine carcinoma; SNB: sentinel lymph node biopsy; TN: triple-negative; TN-PAC: triple-negative pure apocrine carcinoma. | ||||
| Median age, years (min. - max.) | 67 (43 - 94) | 71 (48 - 81) | 64 (43 - 94) | 0.108 |
| Hormonal status, N (%) | ||||
| Pre-menopausal | 5 (9.4) | 3 (12.5) | 2 (6.9) | 0.446 |
| Post-menopausal | 44 (83.0) | 20 (83.3) | 24 (82.8) | |
| NA | 4 (7.6) | 1 (4.2) | 3 (10.3) | |
| Median tumor size, cm (min. - max.) | 2.2 (0.5 - 10.0) | 1.4 (0.5 - 8.0) | 2.1 (1.1 - 10.0) | 0.024 |
| Pathological node status, N (%) | ||||
| Positive | 14 (26.4) | 3 (12.5) | 11 (37.9) | 0.036 |
| Negative | 39 (73.6) | 21 (87.5) | 18 (62.1) | |
| Median Ki-67 index (min. - max.) | 0 - 70 | 5 (0 - 70) | 6.5 (0 - 60) | 0.855 |
| Nuclear grade, N (%) | ||||
| 1 | 12 (22.6) | 6 (25.0) | 6 (20.7) | 0.197 |
| 2 | 14 (26.4) | 10 (41.7) | 4 (13.8) | |
| 3 | 19 (35.9) | 6 (25.0) | 13 (44.8) | |
| NA | 8 (15.1) | 1 (4.2) | 6 (20.7) | |
| Estrogen receptor status, N (%) | ||||
| Positive | 13 (24.5) | 0 (0) | 13 (44.8) | < 0.001 |
| Negative | 40 (75.5) | 24 (100) | 16 (55.2) | |
| Progesterone receptor status, N (%) | ||||
| Positive | 10 (18.9) | 0 (0) | 10 (34.4) | < 0.001 |
| Negative | 43 (81.1) | 24 (100) | 19 (65.6) | |
| HER2 status, N (%) | ||||
| Positive | 11 (20.8) | 0 (0) | 11 (37.9) | < 0.001 |
| Negative | 41 (77.4) | 24 (100) | 17 (58.6) | |
| NA | 1 (1.9) | 0 (0) | 1 (3.5) | |
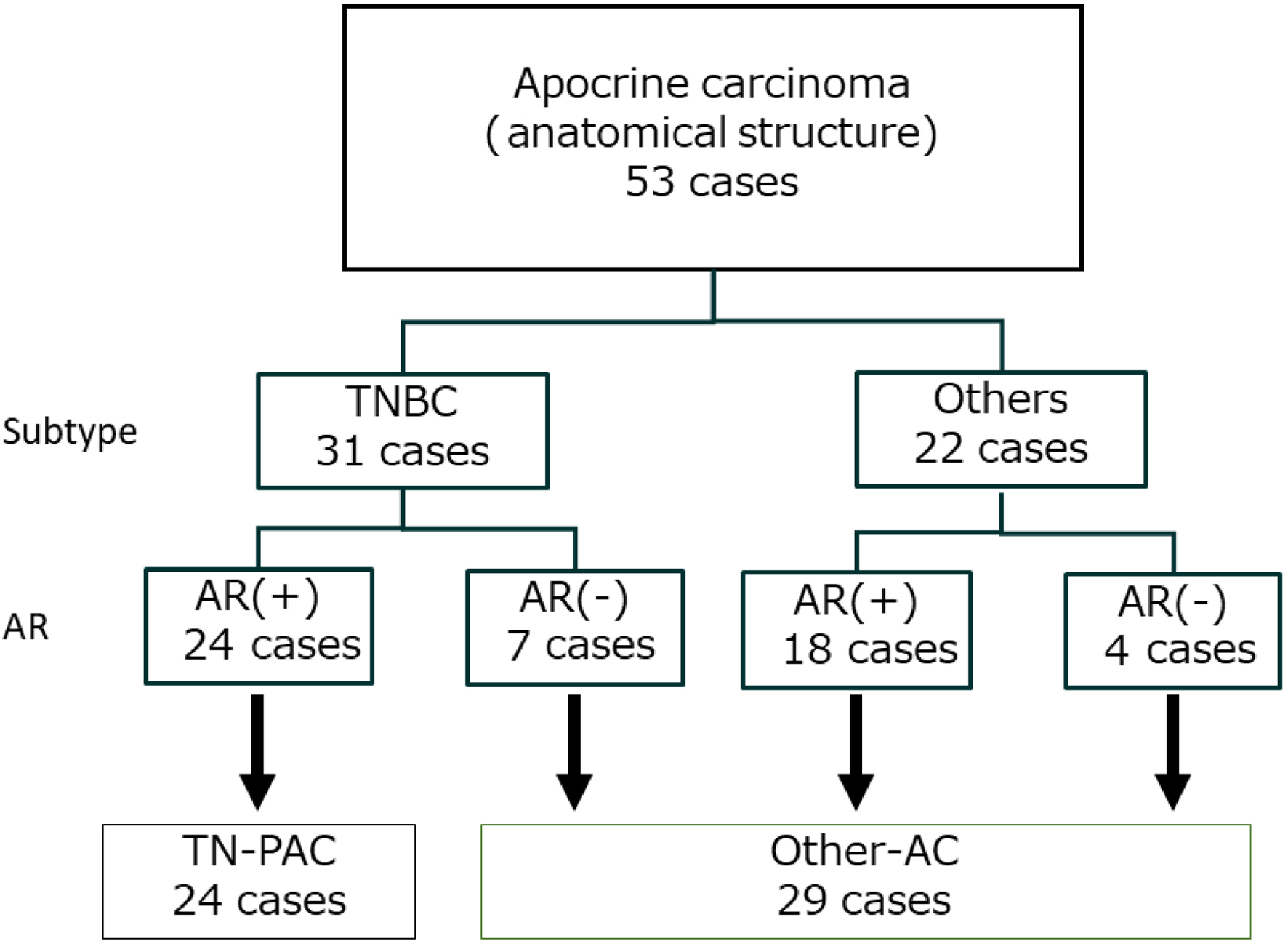
| TN subtype, No. (%) | ||||
| Yes | 31 (58.5) | 24 (100) | 7 (24.1) | < 0.001 |
| No | 22 (41.5) | 0 (0) | 22 (75.9) | |
| AR status, N (%) | ||||
| Positive | 42 (79.2) | 24 (100) | 18 (62.1) | < 0.001 |
| Negative | 11 (20.8) | 0 (0) | 11 (37.9) | |
| Breast cancer stage, N (%) | ||||
| I | 24 (45.3) | 14 (58.3) | 10 (34.5) | 0.157 |
| II | 21 (39.6) | 9 (39.0) | 12 (41.4) | |
| III | 7 (13.2) | 1 (4.2) | 6 (20.7) | |
| IV | 1 (1.9) | 0 (0.0) | 1 (3.4) | |
| Breast surgery, N (%) | ||||
| BCS | 22 (41.5) | 11 (45.8) | 11 (37.9) | 0.588 |
| Mastectomy | 31 (58.5) | 13 (54.2) | 18 (62.1) | |
| Axillary surgery, N (%) | ||||
| SNB | 32 (60.4) | 19 (79.2) | 13 (44.8) | 0.013 |
| Ax | 21 (39.5) | 5 (20.8) | 16 (55.2) | |
| Systemic therapy received, N (%) | ||||
| Yes | 35 (66.0) | 12 (50.0) | 23 (79.3) | 0.038 |
| No | 17 (32.1) | 11 (45.8) | 6 (20.7) | |
| NA | 1 (1.9) | 1 (4.2) | 0 (0.0) | |
| Chemotherapy received, N (%) | ||||
| Yes | 28 (52.8) | 12 (50.0) | 16 (55.2) | 0.525 |
| No | 24 (45.3) | 11 (45.8) | 13 (44.8) | |
| NA | 1 (1.9) | 1 (4.2) | 0 (0.0) | |
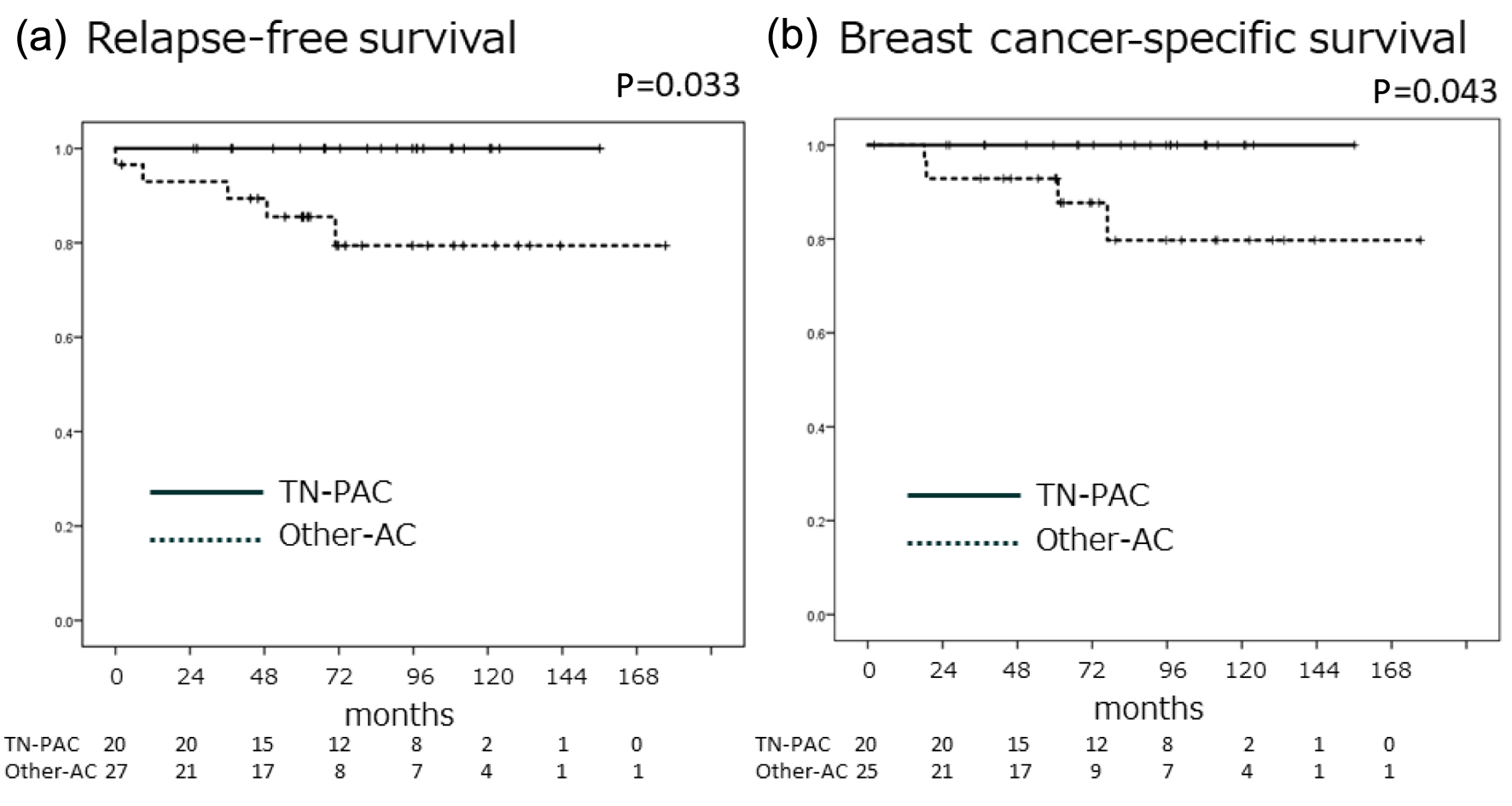
| Relapse, N (%) | 5 (9.4) | 0 (0.0) | 5 (17.2) | 0.041 |
| All-cause mortality, N (%) | 6 (11.3) | 2 (8.3) | 4 (13.8) | 0.678 |
| Breast-cancer mortality, N (%) | 4 (7.5) | 0 (0.0) | 4 (13.8) | 0.081 |