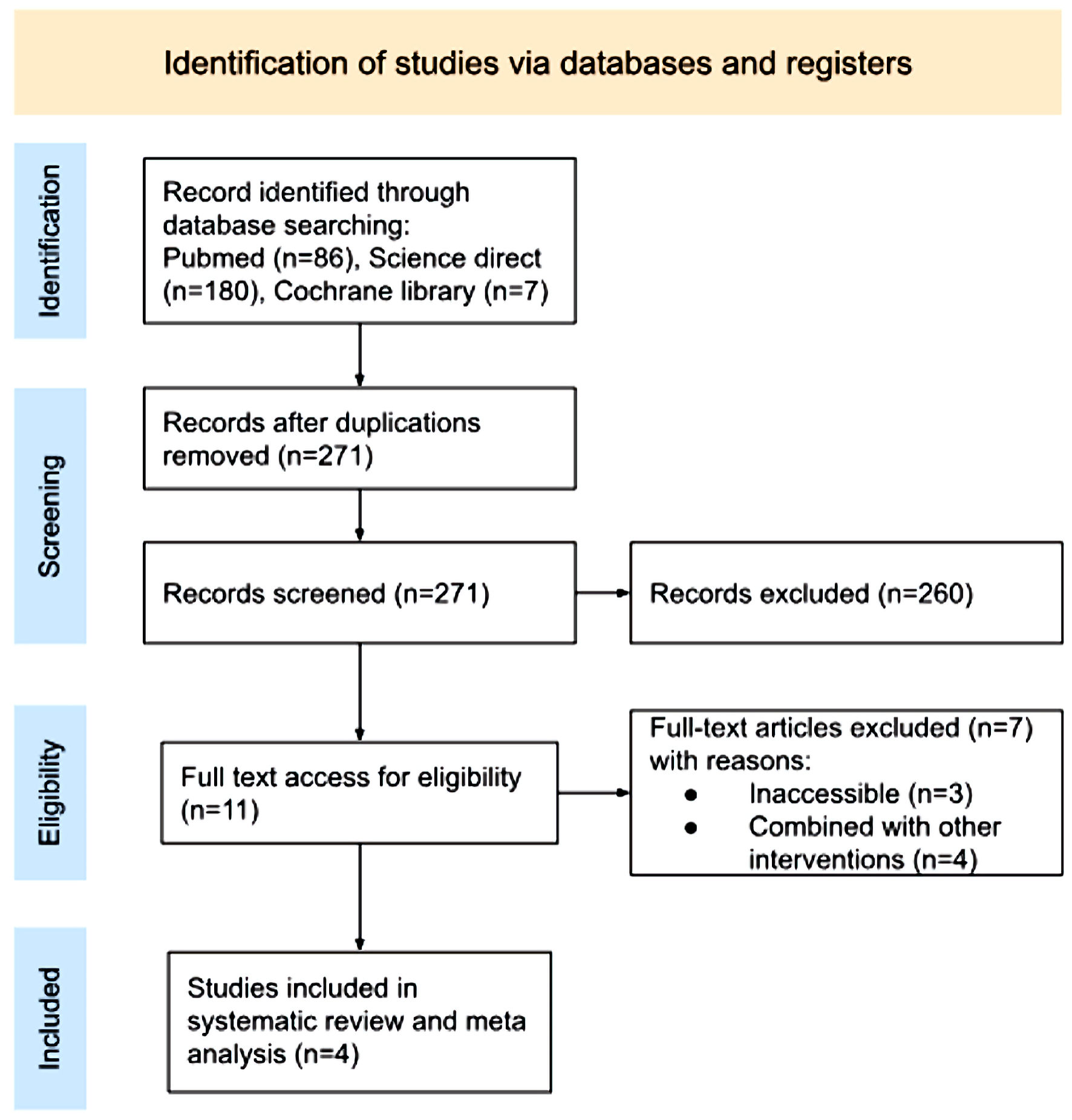
Figure 1. PRISMA 2020 flow diagram. PRISMA: Preferred Reporting Items for Systematic Review and Meta Analyses.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 1, February 2024, pages 72-80
The Addition of Atezolizumab to Chemotherapy in Non-Small Cell Lung Cancer: A Trial-Based Review and Meta-Analysis
Figures

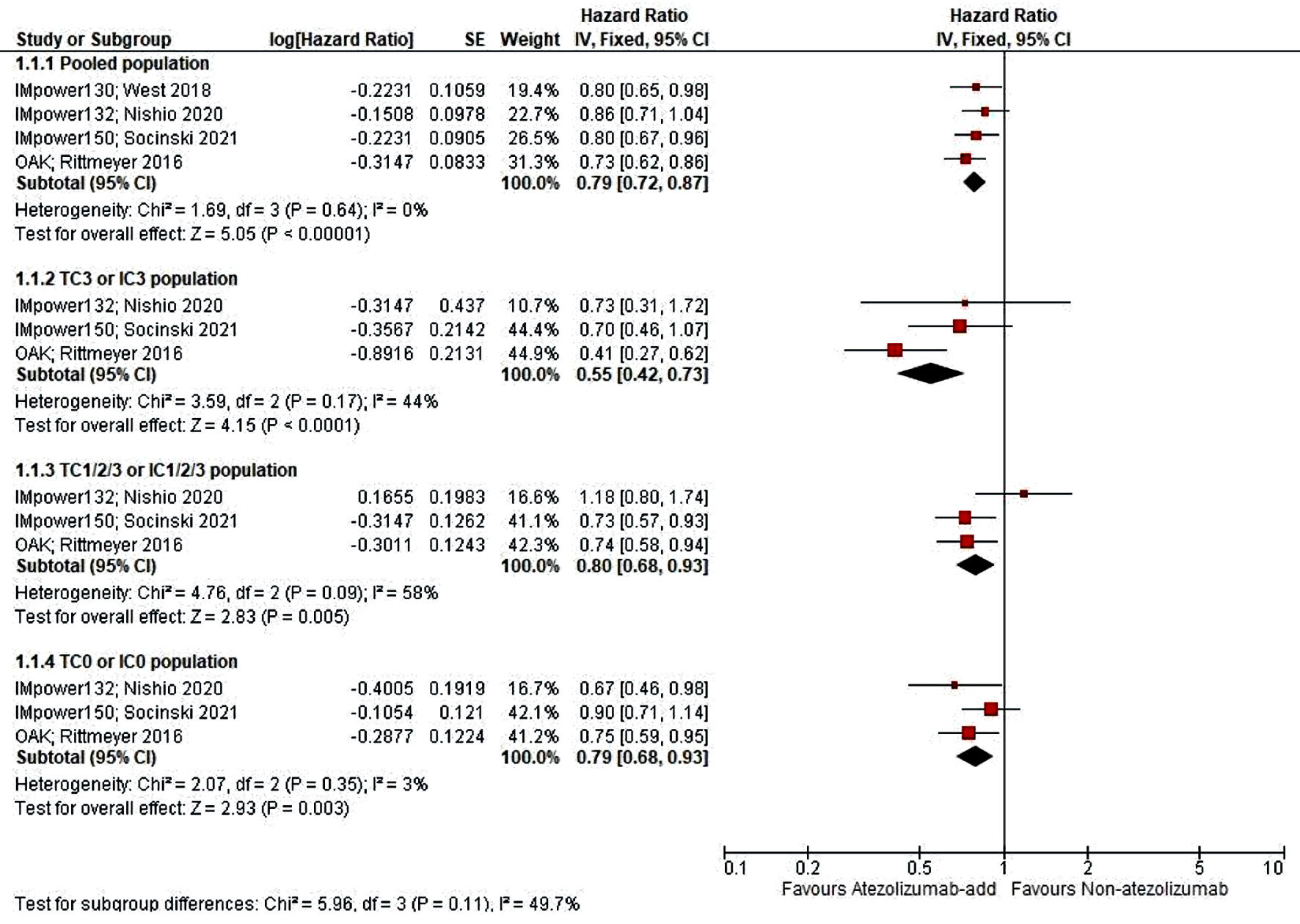
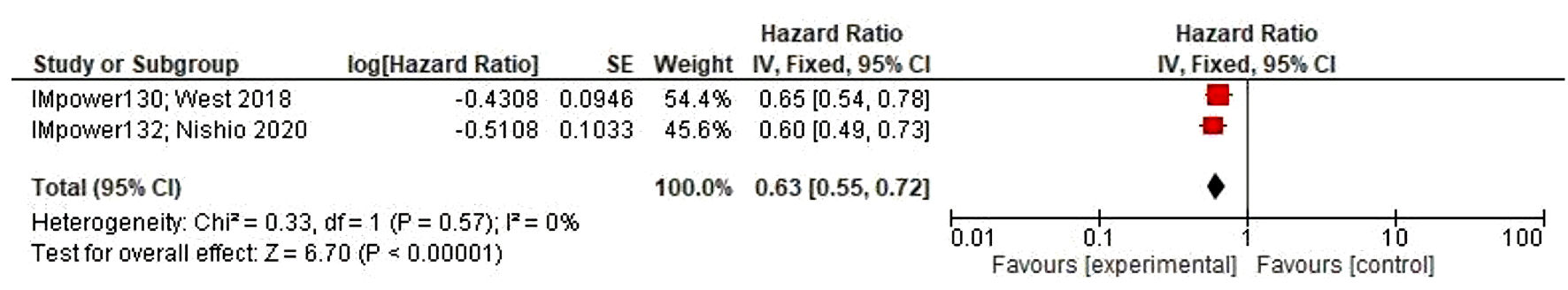
Table
| Study | Criteria for eligibility | Patient/population | Intervention | Comparison | Follow-up | Outcomes | Quality score |
|---|---|---|---|---|---|---|---|
| NSCLC: non-small cell lung cancer; OS: overall survival; PFS: progression-free survival. | |||||||
| Socinski et al, 2021 [11] | ≥18 years old, chemotherapy naive | 802 patients stage IV NSCLC | Atezolizumab: 1,200 mg | Bevacizumab: 15 mg/kg | 32.4 months | Improved OS in the second intervention arm (19 months) than in the control arm (14.7 months). | Jadad score 3: 1) Randomized: yes; 2) Randomized described: yes; 3) Double-blind: no; 4) Blinding described: no; 5) Withdrawals: yes |
| Stage IV metastatic non-squamous NSCLC | Carboplatin: area under the concentration-time curve of 6 mg/mL/min | Carboplatin: area under the concentration-time curve of 6 mg/mL/min | |||||
| Available tumor tissue for biomarker testing | Paclitaxel: 200 mg/m2 (for patients of Asian ethnicity, use 175 mg/m2) | Paclitaxel: 200 mg/m2 (for patients of Asian ethnicity, use 175 mg/m2) | Improved PFS in the first intervention arm (8.4months) than in the control arm (6.8 months). | ||||
| Eastern Cooperative Oncology Group performance status of 0 or 1 | |||||||
| Atezolizumab: 1,200 mg | |||||||
| Bevacizumab: 15 mg/kg | |||||||
| Carboplatin: area under the concentration-time curve of 6 mg/mL/min | |||||||
| Paclitaxel: 200 mg/m2 (for patients of Asian ethnicity, use 175 mg/m2) | |||||||
| Nishio et al, 2021 [10] | ≥ 18 years old | 578 patients stage IV NSCLC | Atezolizumab: 1,200 mg (fixed dose) every 21 days | Cisplatin: 75 mg/m2 every 21 days or | 32.4 months | Improved OS in the intervention arm (18.1 months) than in the control arm (13.6 months). Improved PFS in the intervention arm (7.6 months) than in the control arm (5.2 months). | Jadad score 3: 1) Randomized: yes; 2) Randomized described: yes; 3) Double-blind: no; 4) Blinding described: no; 5) Withdrawals: yes |
| Histologically or cytologically confirmed stage IV non-squamous NSCLC | Cisplatin: 75 mg/m2 every 21 days or | Carboplatin: target area under the concentration-time curve of 6 mg/mL/min every 21 days | |||||
| Treatment-free interval of greater than or equal to 6 months was required if patients had previous chemotherapy or radiotherapy | Carboplatin: target area under the concentration-time curve of 6 mg/mL/min every 21 days | Pemetrexed: 500 mg/m2 every 21 days | |||||
| Eastern Cooperative Oncology Group performance status of 0 or 1 | Pemetrexed: 500 mg/m2 every 21 days | ||||||
| West et al, 2019 [8] | ≥ 18 years or older, and had histologically or cytologically confirmed stage IV non-squamous NSCLC | 723 patients stage IV NSCLC | Atezolizumab: 1,200 mg/m2 intravenously every 3 weeks | Carboplatin: area under the curve 6 mg/mL/min every 3 weeks | 18.5 months | Improved OS in the intervention arm (18.6 months) than in the control arm (13.9 months). Improved PFS in the intervention arm (7 months) than in the control arm (5.5 months). | Jadad score 3: 1) Randomized: yes; 2) Randomized described: yes; 3) Double-blind: no; 4) Blinding described: no; 5) Withdrawals: yes |
| Received no previous chemotherapy for stage IV disease | Carboplatin: area under the curve 6 mg/mL/min every 3 weeks | Nab-paclitaxel: 100 mg/m2 intravenously every week | |||||
| Eastern Cooperative Oncology Group performance status of 0 or 1 | Nab-paclitaxel: 100 mg/m2 intravenously every week | ||||||
| Rittmeyer et al, 2017 [9] | ≥18 years old | 1,225 patients stage IIIB-IV NSCLC | Atezolizumab: 1,200 mg/m2 intravenously every 3 weeks | Docetaxel: 75 mg/m2 every 3 weeks | 21 months | Improved OS in intervention arm (13.8 months) than in control arm (9.6 months). | Jadad score 3: 1) Randomized: yes; 2) Randomized described: yes; 3) Double-blind: no; 4) Blinding described: no; 5) Withdrawals: yes |
| Patients had received one to two previous cytotoxic chemotherapy regimens (≥ 1 platinum-based combination therapy) for stage IIIB or IV NSCLC | |||||||
| Eastern Cooperative Oncology Group performance status of 0 or 1 | |||||||