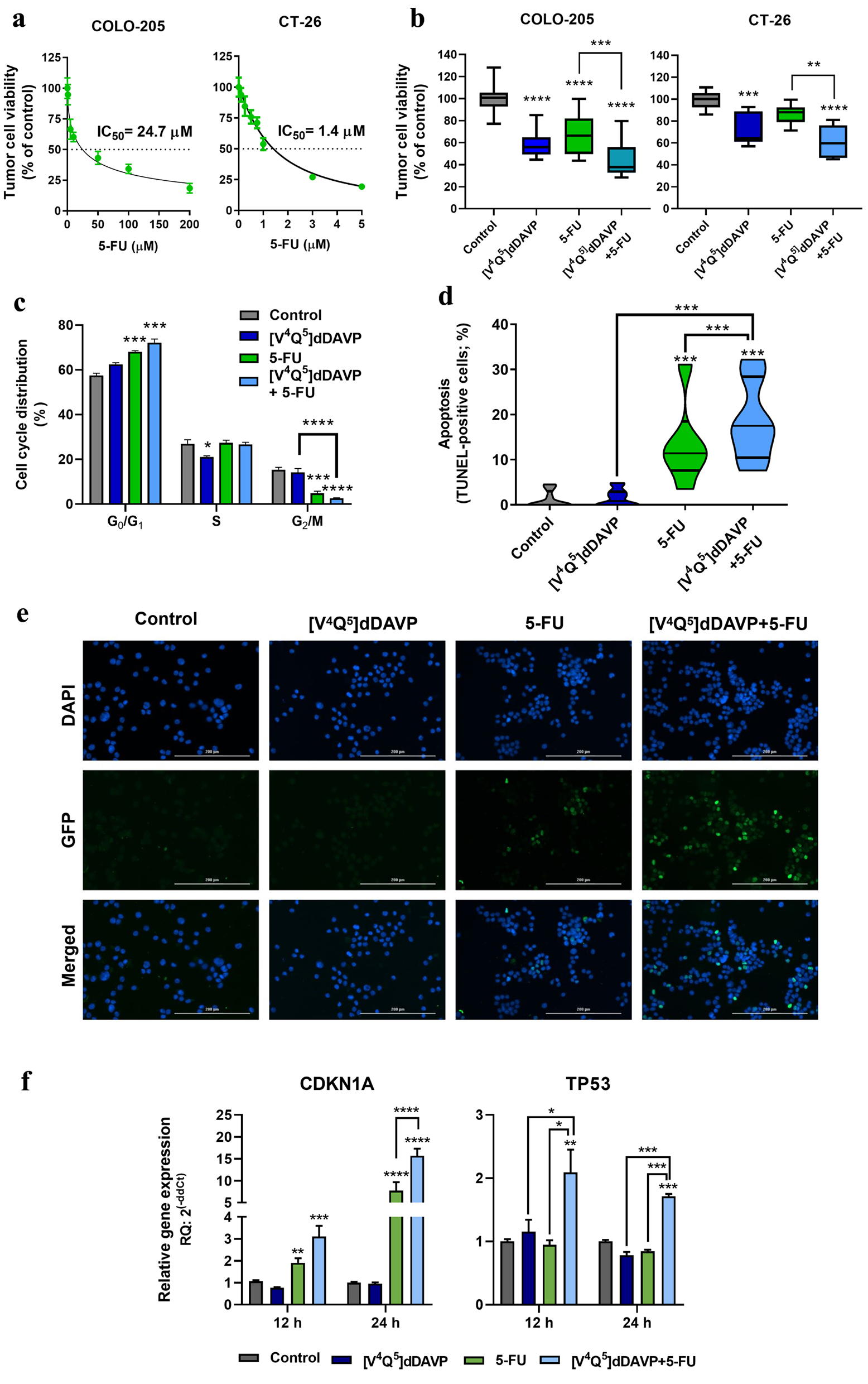
Figure 1. In vitro cytostatic activity of [V4Q5]dDAVP in combination with 5-fluorouracil on colorectal cancer cells and its impact on apoptosis induction and cell cycle progression. (a) Cytotoxic activity of 5-fluorouracil on COLO-205 (left) and CT-26 (right) colorectal cancer cells and IC50 calculation after a 72-h exposure to chemotherapeutic agent. (b) Effect on colorectal cancer cell viability of [V4Q5]dDAVP (1 µM) addition to 5 µM 5-fluorouracil for COLO-205 cells (left) or 0.5 µM for CT-26 cells (right). Direct cytotoxic/cytostatic effects on cancer cells were assessed by the metabolic MTS assay. (c) Cell cycle phase distribution evaluated by flow cytometry for COLO-205 cells after 24 h treatment with [V4Q5]dDAVP (1 µM), 5-fluorouracil (5 µM) or dual combined therapy. (d) Percentage of apoptotic cells after 48 h treatment with [V4Q5]dDAVP (1 µM), 5-fluorouracil (5 µM) or its combination in human colorectal cancer cell cultures. Apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL). (e) Representative images of TUNEL labeling in human COLO-205 cell cultures under different treatments (× 200 magnification. Scale bar = 200 µm). (f) Relative gene expression of cyclin-dependent kinase inhibitor p21 (CDKN1A) and the tumor suppressor p53 (TP53) by quantitative reverse transcription polymerase chain (qRT-PCR) in colorectal cancer cells after 24 h treatment with [V4Q5]dDAVP and 5-fluorouracil, in combination or as monotherapies. Data are presented as mean ± standard error of mean (SEM) (a, c, f), box and whiskers with minimum to maximum values (b) or violin plots (d) and are representative of at least two or three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
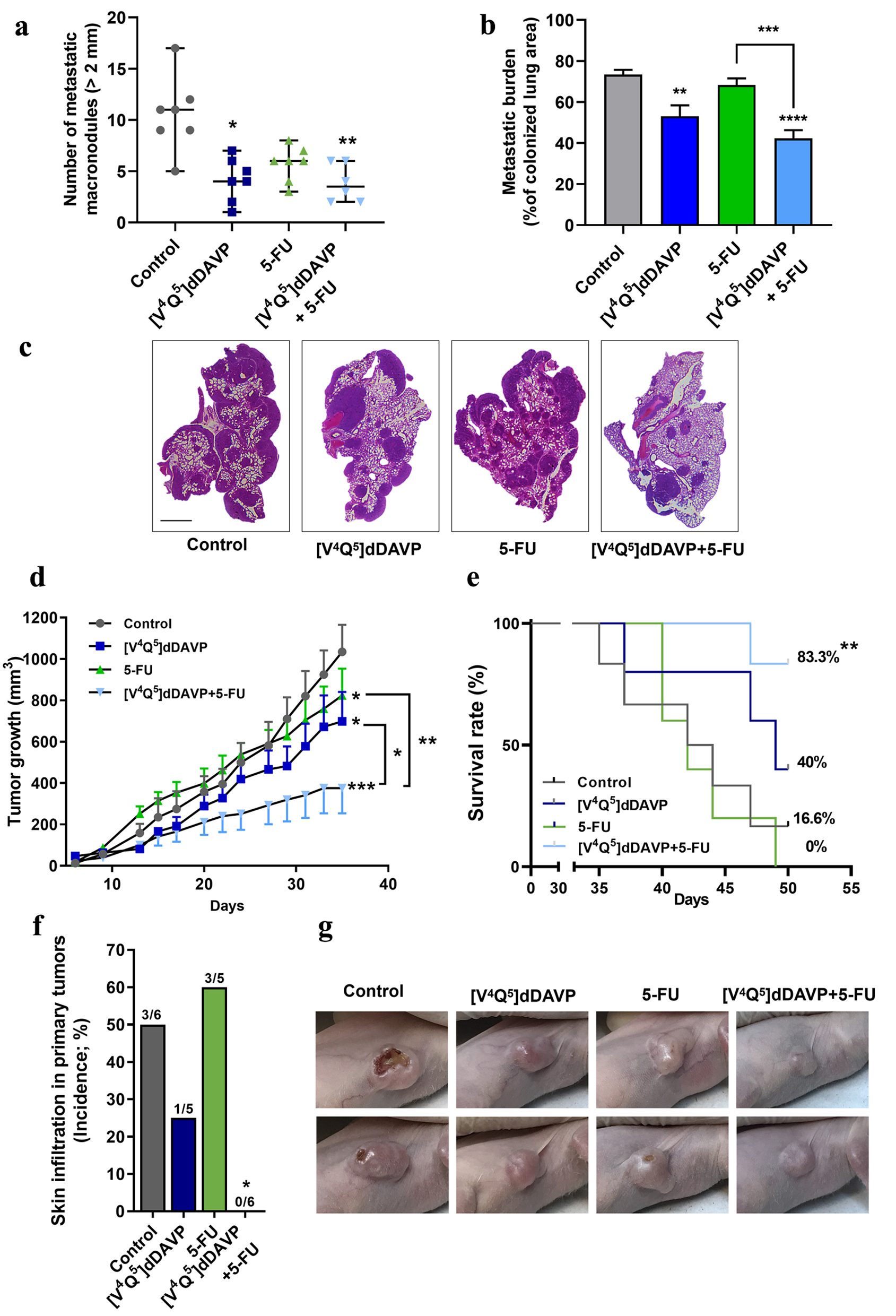
Figure 2. In vivo combinational effects of [V4Q5]dDAVP addition to low doses of 5-fluorouracil on colorectal cancer metastatic spread and tumor growth. Effects of combined therapy on CT-26 cell metastatic dissemination to lung (a, b and c) and COLO-205 xenograft progression (d, e, f and g) are shown. (a) Quantification of surface macrometastatic (> 1 mm of diameter) pulmonary lesions 21 days after highly metastatic CT-26 cells were injected intravenously (IV) in BALB/c mice. Animals were treated with [V4Q5]dDAVP (0.3 µg/kg IV) and 5-fluorouracil (50 mg/kg intraperitoneal (IP)), alone or in combination. (b) Metastatic burden in lungs was also assessed and quantified by computer-assisted histopathological analysis as area of CT-26 lung metastasis per total lung area. (c) Representative photographs of hematoxylin and eosin (H&E)-stained sections of lungs colonized by CT-26 metastatic cells from control mice receiving saline solution, [V4Q5]dDAVP or 5-fluorouracil alone, or [V4Q5]dDAVP plus 5-fluorouracil dual treatment. Scale bar = 2 µm. (d) Assessment of primary COLO-205 xenograft progression in nude mice. Curves representing tumor volume over time in mice receiving saline solution, [V4Q5]dDAVP (0.3 µg/kg IV), 5-fluorouracil (80 mg/kg IP) or its combination are shown. Statistical analysis was conducted on tumor growth rates calculated from day 10 to 31. (e) Effect of [V4Q5]dDAVP treatment, alone or co-administered with 5-fluorouracil-based chemotherapy, on survival of mice bearing COLO-205 xenografts. Kaplan-Meier survival plot for different experimental groups. (f) Incidence of skin infiltration in mice bearing COLO-205 xenografts treated with saline solution, [V4Q5]dDAVP, 5-fluorouracil (50 mg/kg IP) or dual concomitant therapy. (g) Representative high-resolution photographs of nude mice bearing COLO-205 primary tumors belonging to the different experimental groups were taken at day 29 of the colorectal cancer progression protocol and are representative of five or six animals per experimental group. Data are presented as scatter dot blots showing median with range (a), mean ± standard error of mean (SEM) (b, d) or percentages (e, f). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.

