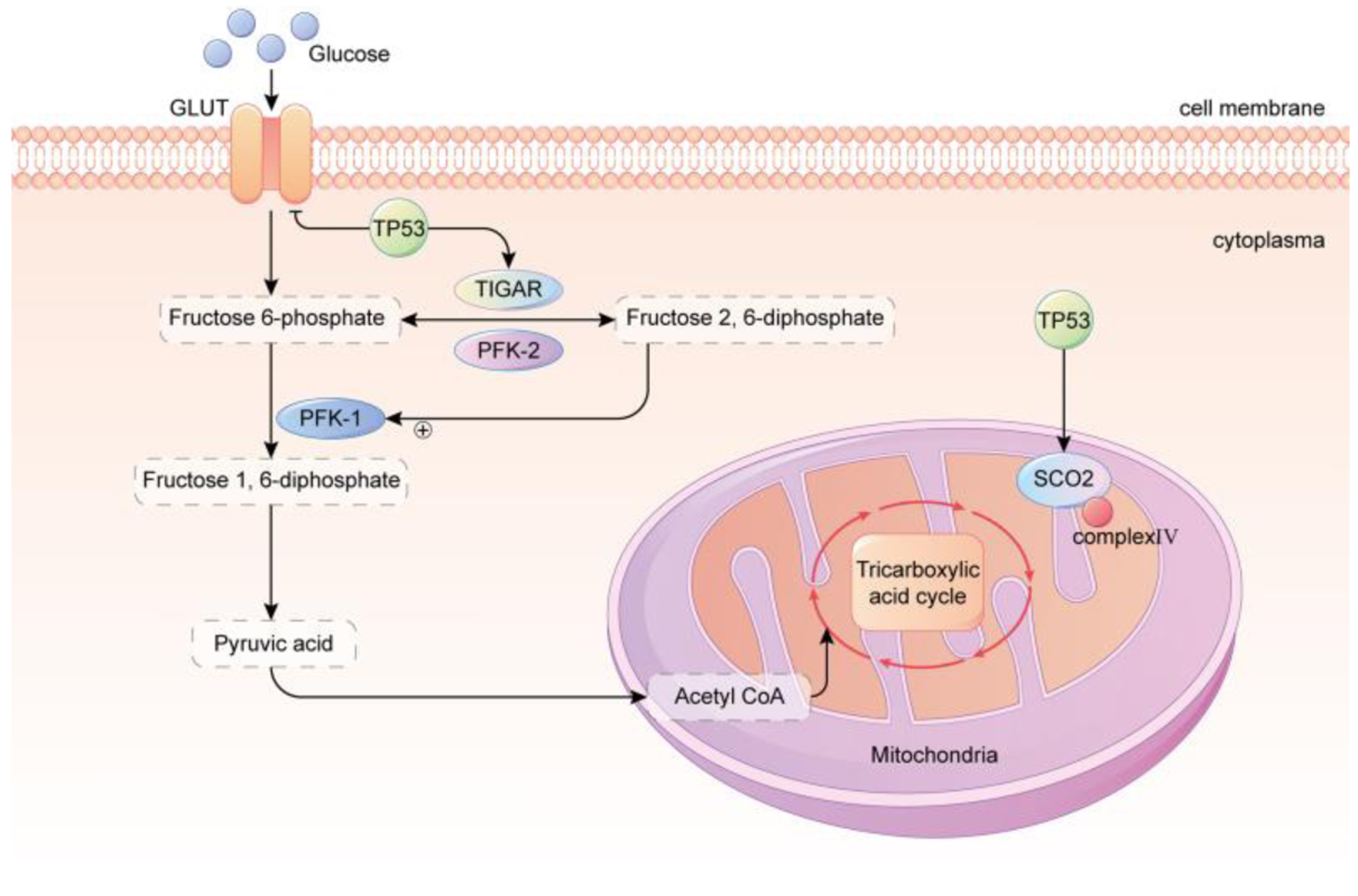
Figure 1. TP53 regulated glycolysis inhibition and OXPHOS promotion. GLUT, which is primarily responsible for transporting glucose, is downregulated by TP53. TP53 upregulates the levels of TIGAR and the mitochondrial respiration chain complex IV subunit SCO2, resulting in a reduction of glycolysis and an enhancement of OXPHOS in the mitochondria. In conclusion, the metabolic phenotype of cancer cells is strongly correlated with the TP53 status. TP53 of wild type possesses robust mitochondrial oxidative phosphorylation and glycolytic reserve capacities, and cells maintain metabolic diversity. In contrast, mutant TP53 cells rely heavily on glycolysis, have a markedly diminished mitochondrial and glycolytic reserve, and operate close to capacity under baseline conditions. GLUT: glucose transporter; TP53: tumor suppressor 53; TIGAR: TP53-induced glycolysis and apoptosis regulator; PFK-1: phosphofructokinase-1; PFK-2: phosphofructokinase-2; SCO2: synthesis of cytochrome c oxidase 2.
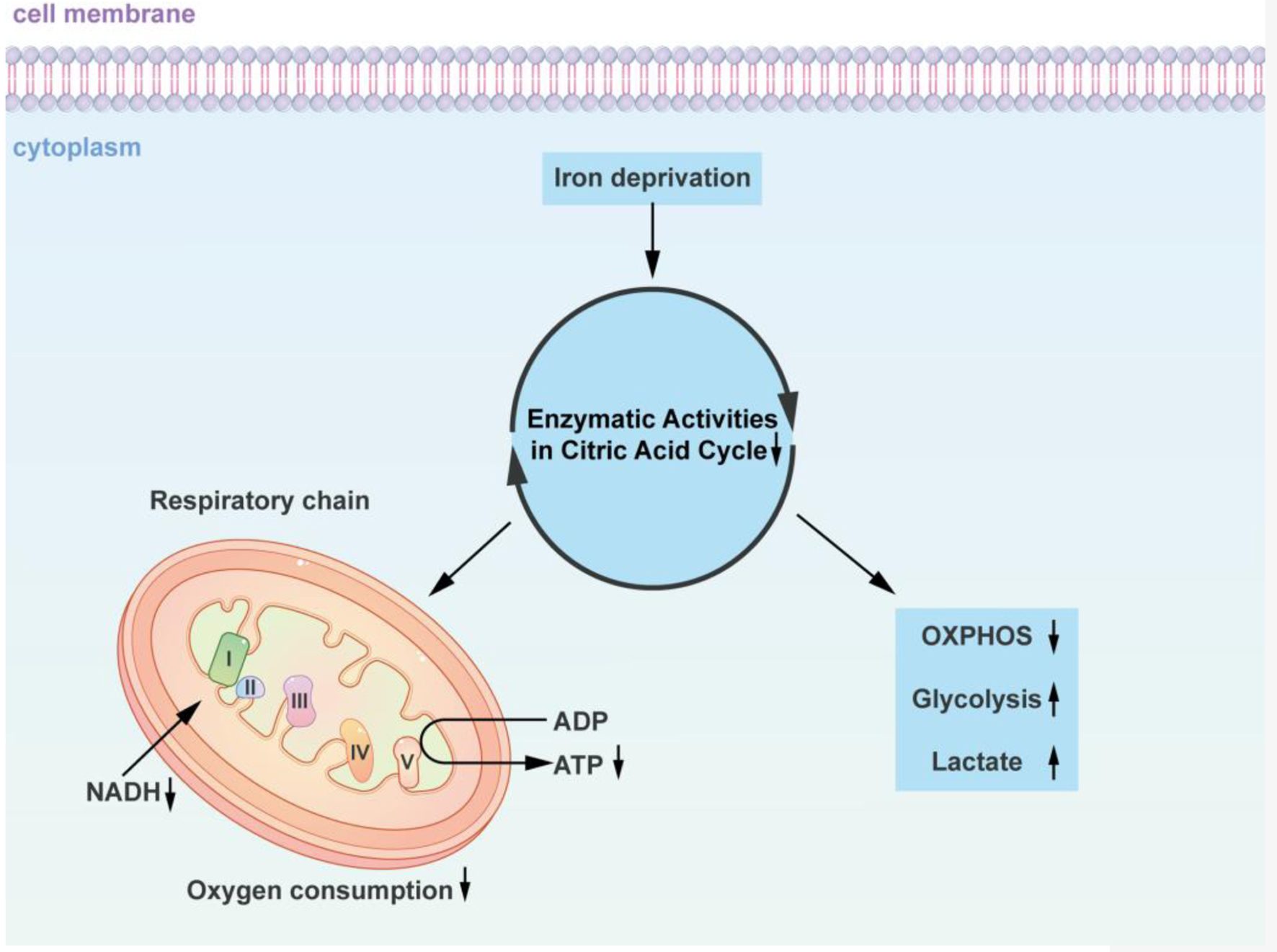
Figure 2. Iron-dependent alterations in cellular energy metabolism: impact on the citric acid cycle and oxidative phosphorylation. The diagram concisely depicts how iron-deficient cells alter the citric acid cycle as well as oxidative phosphorylation. Using iron chelating agents or alternative approaches to reduce the iron content in the cell culture environment decreased the cells’ oxygen consumption, which in turn decreased the citrate acid cycle and NADH enzymatic activities. Iron deficiency promotes glucose and glycolysis utilization for energy, as well as a decline in the oxidation of fatty acids in the respiratory chain of the mitochondria and a decrease in ATP production. Similar metabolic changes are induced by iron deficiency and oxygen deficiency in the absence of iron. Insufficiency in iron and oxygen promotes glucose utilization and glycolysis for energy, increases lactic acid production, and inhibits mitochondrial respiratory chain fatty acid oxidative phosphorylation. NADH: nicotinamide adenine dinucleotide; ADP: adenosine diphosphate; ATP: adenosine triphosphate; OXPHOS: oxidative phosphorylation.
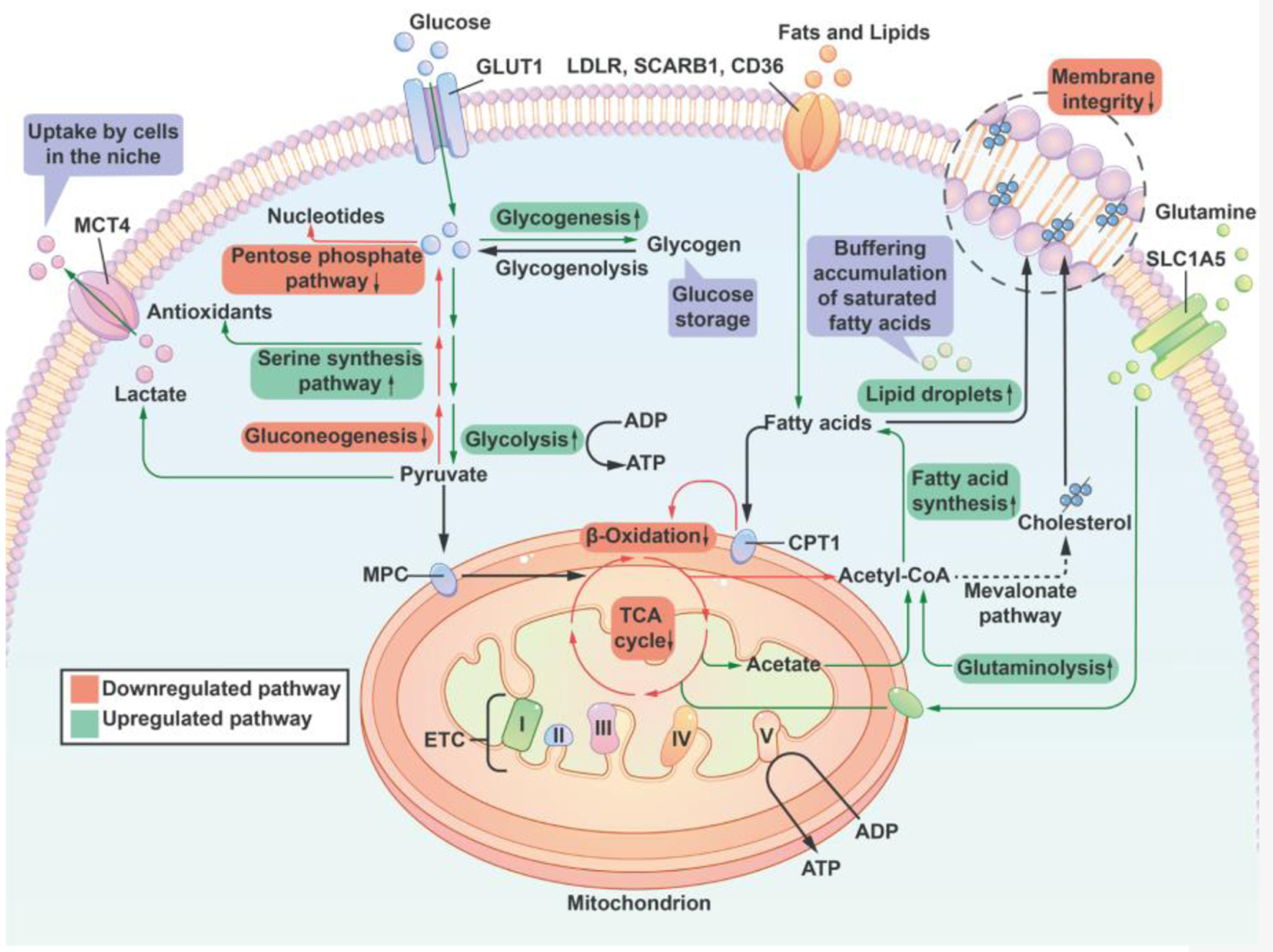
Figure 3. A summary of lipid and sugar metabolic pathways that are regulated by the supply of oxygen. Cells reduce the activity of the pentose phosphate pathway and nucleotide synthesis to compensate for a decrease of antioxidant capacity thereby offering cell protection. Additionally, hypoxia promotes glycogenesis to provide reserves of energy for cells to endure long-term duress. Under conditions of hypoxia, the TCA cycle is inhibited, and Ac-CoA, which is essential for fatty acid synthesis, is primarily supplied by increased glutamine uptake and metabolism. At the same time, the oxidative phosphorylation catabolism of fatty acids is inhibited, resulting in an increase in the uptake of lipids from the environment. Hypoxia inhibits the synthesis of unsaturated fatty acids (a reduced activity of stearoyl-CoA desaturase due to insufficient oxygen). To counteract the potential lipotoxicity caused by the accumulation of saturated fat, cells increased their intake of unsaturated lipids from the external environment and the development of lipid droplets in order to maintain the structural integrity of the cell membrane. Lipid droplets can serve as a buffer for lipid species that are saturated in order to stabilize the cell membrane. I, II, III, IV: respiratory complex I-IV; ETC: electron transport chain; CPT1: carnitine palmitoyl transferase 1; GLUT1: glucose transporter 1; MCT4: monocarboxylic acid transporter 4; LDLR: low-density lipoprotein receptor; MPC: mitochondrial pyruvate carrier; SLC1A5: member of solute carrier family 1 (neutral amino acid transporter) 5; SCARB1: scavenger receptor B1; TCA: tricarboxylic acid; Ac-CoA: Acetyl CoA.


