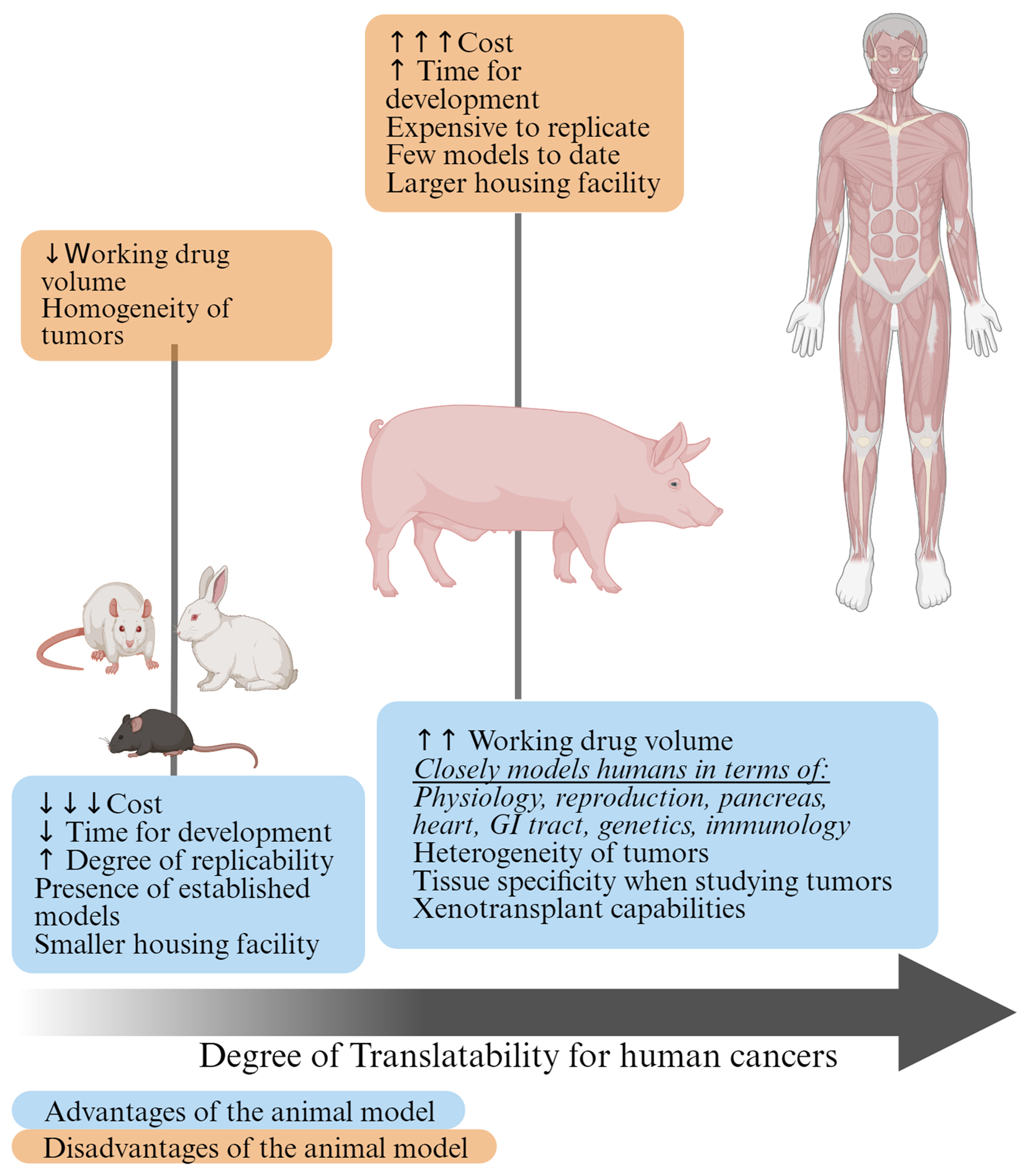
Figure 1. Advantages and disadvantages of using small and large preclinical cancer models. Given the similarities between pigs and humans, the pigs lie closer on the scale of translatability to humans compared to small animal models.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 15, Number 2, April 2024, pages 149-168
Pigs: Large Animal Preclinical Cancer Models
Figures

Table
| Type of cancer/intervention methods | Method | General comments | References |
|---|---|---|---|
| Glioma/glioblastoma | U87 GM/G-6 (primary culture of GBM) | [16-18] | |
| Spinal cord glioma | Lentiviral vectors | Vector 1: PDGF-B-IRES-eGFP Vector 2: HRASG12V-IRES-mPlum Vector 3: pLKO1 backbone + shRNA targeting p53 | [18] |
| Microbrachytherapy | 165Ho siloxane particles | U87 cells for tumorigenesis | [19] |
| Osteosarcoma | Fetal fibroblasts Nuclear and Embryo transfer generating TP53+/+, TP53R167H/+, and TP53R167H/R167H | [20] | |
| MSCs xenografted AdCre inducible CAG-LSL-TP53R167H homozygous-KRASG12D heterozygous-MYC model | [21] | ||
| MSCs Cre recombinase inducible TP53R167H followed by somatic cell transfer | [22, 62] | ||
| Hematological malignancies | Partial T-cell depleted adult pig bone marrow injected into the intrahepatic portion of the umbilical vein of fetal swine via transuterine injection | Demonstrated chimerism and GVHD MHG-MHC SLAcc (class Ic/IIc) | [23] |
| NK cells killed human cancer cell lines (pancreatic, melanoma and CML) in vitro in SCID pig model | Line of Yorkshire pigs that was selected for increased feed efficiency at Iowa State University | [24] | |
| Naturally occurring CML in MGH MHC pigs | [25] | ||
| HCT (SCF/IL3 mobilized) injected into MGH MHC pigs + total body/thymic irradiation + T-cell depletion + cyclosporine A monotherapy | MGH MHC pigs | [26] | |
| T- B- NK- SCID pig model by generating ART-/- IL2RG-/Y with CRISPR/Cas9 directed mutagenesis | [27] | ||
| Colorectal cancer and inflammatory bowel disease | CRISPR/Cas9-mediated excision of the 93-bp fragment containing the ARE and CDE1 element + microinjecting guide RNA plasmids into in vitro fertilized porcine oocytes followed by laparoscopic embryo transfer | TNFΔARE pigs | [28] |
| Gene-targeted cloned pigs carrying translational stop signals in the APC gene at codons 1061 and 1311 | [29] | ||
| CRISPR/Cas9 based LGR5-H2B-GFP model | OLFM4 expression (similar to human small intestine and colon) | [30] | |
| Pancreatic cancer | AdCre CAG-LSL-KRASG12D-TP53R167H into the body of the pancreas or into the pancreatic duct pushed towards the pancreas | Sus scrofa pigs | [31] |
| AdCre CAG-LSL-KRASG12D-TP53R167H Oncopig used with core biopsy of pancreas + incubation with AdCre then mixture injected back into pancreas | Oncopigs from University of Missouri National Swine Resource and Research Center | [40] | |
| Primary porcine fibroblasts with murine Pdx-1 promoter overexpression of oncogene cassette containing MYC, KRASG12D and SV40 LT + an inducible negative regulator protein (rtTR-KRAB) via somatic cell nuclear transfer | Gottingen-Ellegaard minipig | [32] | |
| Transgenic Oncopigs (CAG-KRASG12D-IRES-TP53R167H) Injection Technique 1: Main duct + parenchyma Injection Technique 2: Connecting lobe | Oncopigs from University of Missouri National Swine Resource and Research Center | [33] | |
| Hepatocellular carcinoma | AdCre in vitro expression of KRASG12D and TP53R167H | Minnesota Minipig sire and Yorkshire dams and heterozygous for the transgene | [34, 35] |
| Transarterial alcohol injection | Model for cirrhosis development Sus scrofa | [34, 36] | |
| AdCre KRASG12D TP53R167H Oncopig + CRISPR/Cas9-mediated ko ARID1A + AXIN1 | Orthotopic implantation of ko HCC cell in Oncopig model | [37, 38] | |
| Prevention of liver precancerous and cancerous lesions | Model of hereditary tyrosinemia type-1 treatment with in vivo lentiviral vector targeting human fumarylacetoacetate hydrolase transgene | Fumarylacetoacetate hydrolase-/- via somatic cell transfer | [39] |
| Immune therapy | Thermal ablation methods | Ex vivo pig livers | [41-43] |
| Vaccine-induced peptide-specific cytotoxic T-cell responses | Outbred Danish Landrace/Yorkshire/Duroc pigs | [46] | |
| Porcine kidney fibroblasts from male Gottingen minipig and transfected with IGH-γ1-γ4 and IGK expression vector. Followed by somatic cell nuclear transfer. | Humanized Gottingen minipigs for toxicological testing of therapeutic recombinant antibodies | [47] | |
| CRISPR/Cas9 system used to induce deletions within exon 4 of porcine TRDC gene. Electroporation to transfect CRISPR/Cas9 plasmids + Intracytoplasmic microinjection + somatic cell nuclear transfer | TRDC-knockout German Landrace pigs lacking γδ T cells | [48] | |
| Preclinical testing of immunotherapies for tumor directed cytotoxicity | Oncopig model | [49] | |
| Autologous hepatocyte derived cell lines transformed using AdCre + transarterial embolization of liver cancer | Oncopig model | [44] | |
| Xenotransplantation | Oncopig model | [50-53] | |
| Histotripsy | [45] | ||
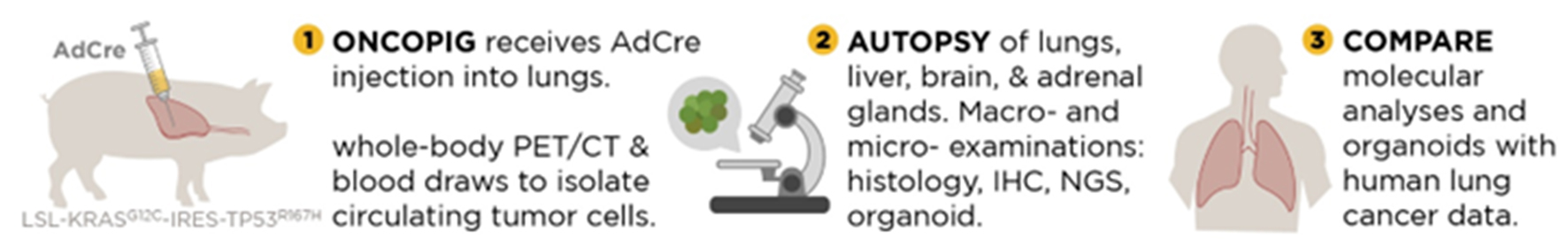
| Lung cancer Potential interventions for lung cancer | pRosa26-iCas9 pigs by intra-nasal delivery | Chinese Bama mini-pigs | [60] |
| Endovascular and percutaneous (biopsy plus incubation with AdCre ex vivo followed by inoculation) delivery | [61] | ||
| Microwave ablation | Oncopigs | [54-56] | |
| Conventional vs. cone beam CT to monitor microwave ablation | [57] | ||
| Xenogeneic cross-circulation to support human donor | [58, 59] |