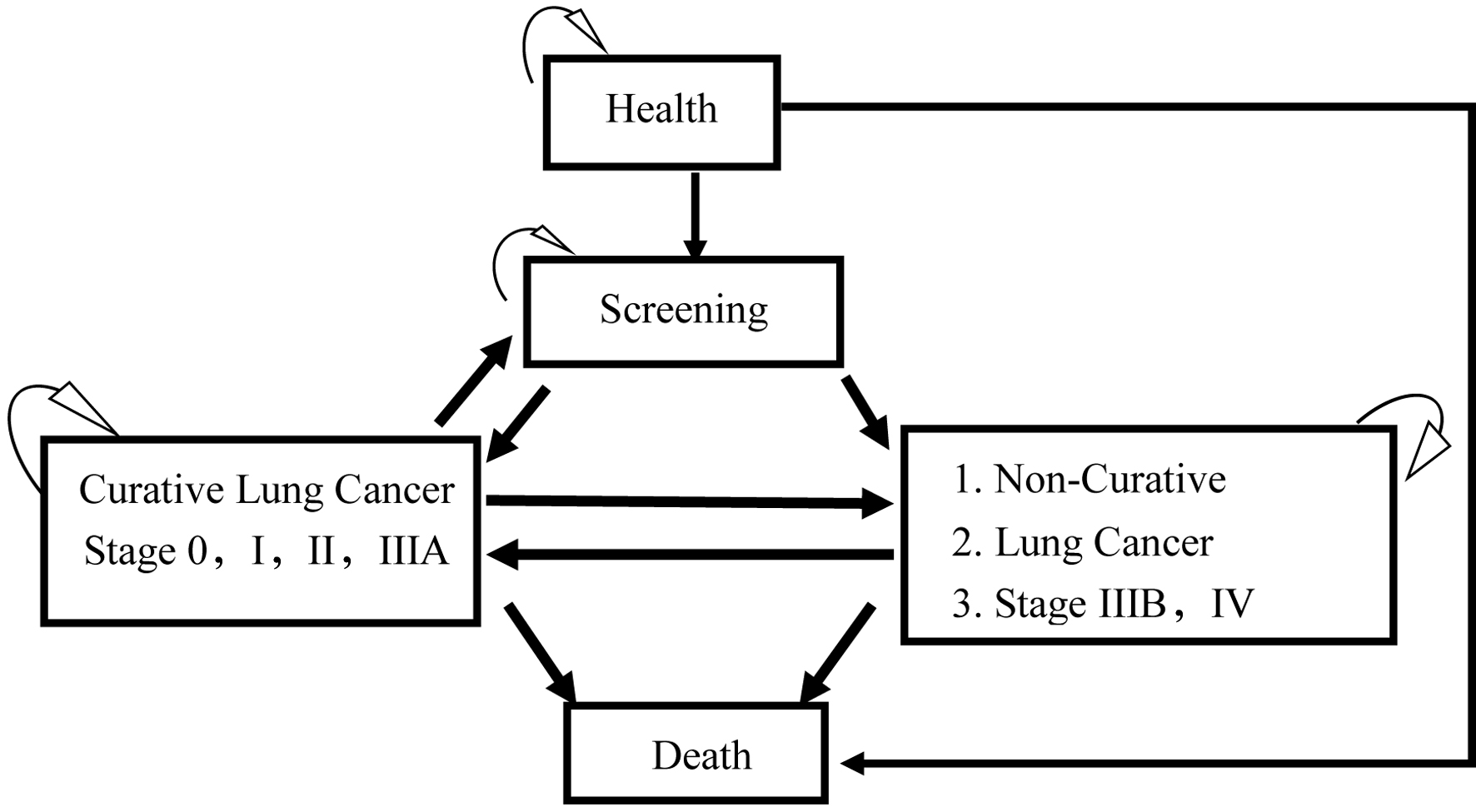
Figure 1. The Markov model of cost-effectiveness analysis of low-dose computed tomography (LDCT) and chest X-ray (CXR) screenings for lung cancer in high-risk populations.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 550-561
Cost-Effectiveness of Low-Dose Computed Tomography Screenings for Lung Cancer in High-Risk Populations: A Markov Model
Figures

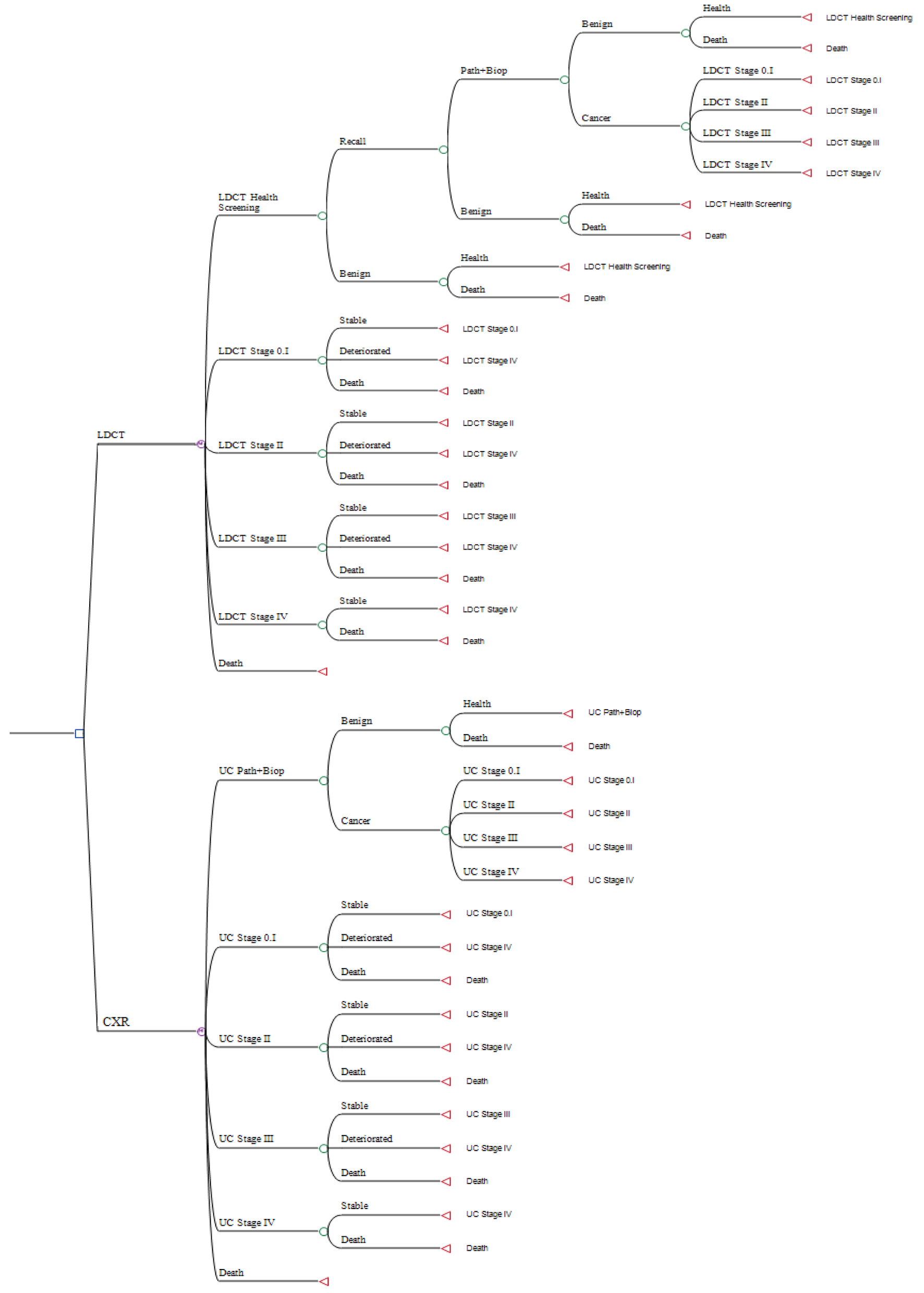
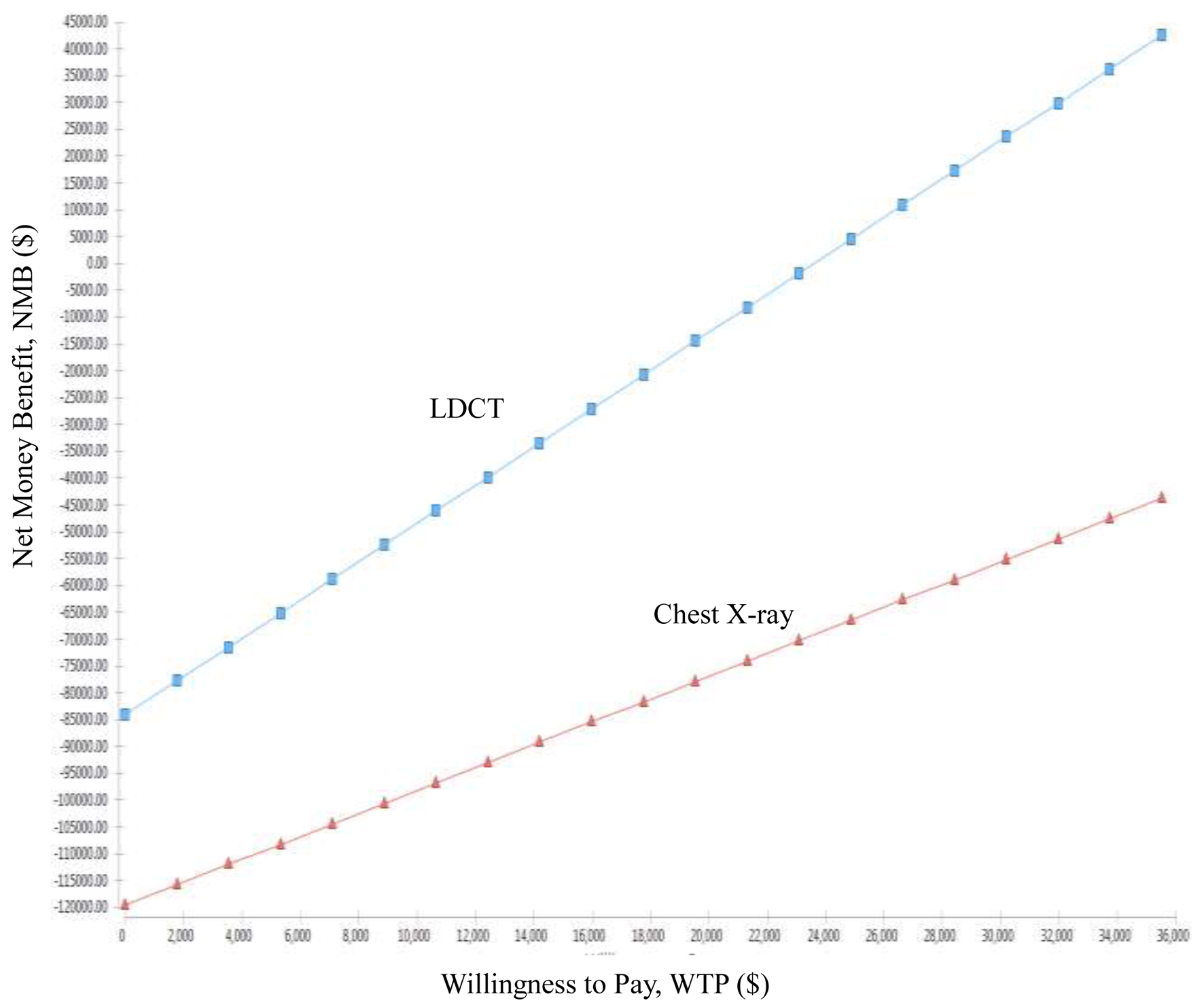
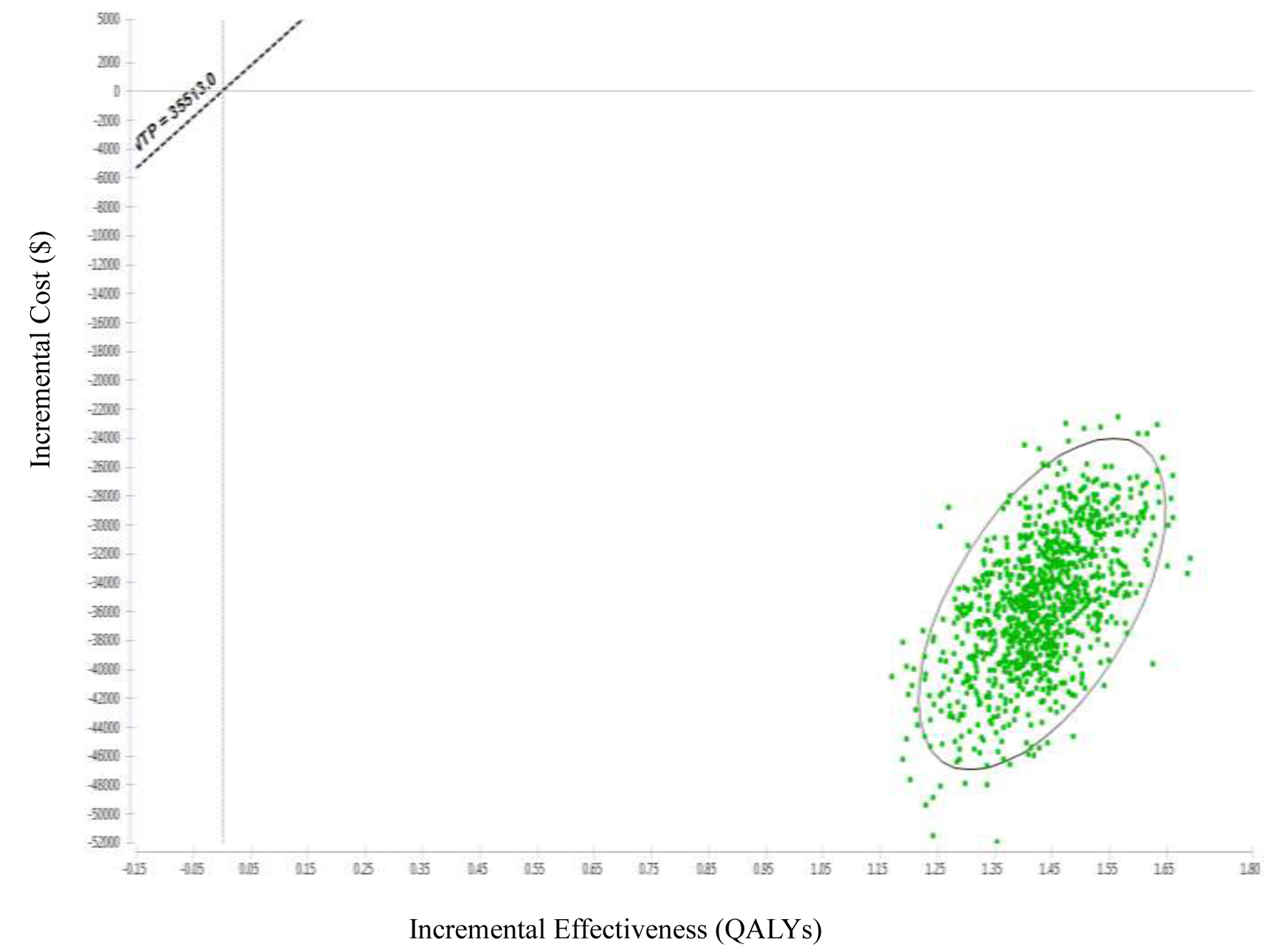
Tables
| Variables | Values | Distribution | Reference |
|---|---|---|---|
| CXR: chest X-ray; HPA: Health Promotion Administration; LDCT: low-dose computed tomography; QALYs: quality-adjusted life years. | |||
| Probability | |||
| LDCT false positive | 96.4% | β | Aberle et al [13] |
| LDCT cancer detection rate | 24.2% | β | Aberle et al [13] |
| CXR false positive | 94.5% | β | Aberle et al [13] |
| CXR cancer detection rate | 6.9% | β | Aberle et al [13] |
| Lung cancer survival rate stage 0-I | 90% | β | HPA [10] |
| Lung cancer survival rate stage II | 60% | β | HPA [10] |
| Lung cancer survival rate stage III | 30% | β | HPA [10] |
| Lung cancer survival rate stage IV | 10% | β | HPA [10] |
| LDCT lung cancer detection rate stage 0-I | 40% | β | Wood et al [15] |
| LDCT lung cancer detection rate stage II | 26% | β | Wood et al [15] |
| LDCT lung cancer detection rate stage III | 12% | β | Wood et al [15] |
| LDCT lung cancer detection rate stage IV | 22% | β | Wood et al [15] |
| CXR lung cancer detection rate stage 0 | 0-4.2% | β | Snowsill et al [16], HPA [10] |
| CXR lung cancer detection rate stage I | 18.0-29.1% | β | Snowsill et al [16], HPA [10] |
| CXR lung cancer detection rate stage II | 4.3-8% | β | Snowsill et al [16], HPA [10] |
| CXR lung cancer detection rate stage III | 12.3-21% | β | Snowsill et al [16], HPA [10] |
| CXR lung cancer detection rate stage IV | 50.1-53% | β | Snowsill et al [16], HPA [10] |
| Cost ($), per cycle: 2 years | |||
| LDCT screening examination | 200 | γ | HPA [10] |
| Treatment for lung cancer stage I | 38,527 (36,546 - 40,968) | γ | Yang et al [17] |
| Treatment for lung cancer stage II | 48,262 (44,656 - 53,412) | γ | Yang et al [17] |
| Treatment for lung cancer stage III | 38,201 (31,111 - 45,191) | γ | Yang et al [17] |
| Treatment for lung cancer stage IV | 26,581 (26,054 - 27,131) | γ | Yang et al [17] |
| Utility (QALYs) | |||
| Lung cancer stage I | 0.76 | β | Yang et al [17] |
| Lung cancer stage II | 0.37 | β | Yang et al [17] |
| Lung cancer stage III | 0.24 | β | Yang et al [17] |
| Lung cancer stage IV | 0.09 | β | Yang et al [17] |
| Healthy adults | 1 | Assumption | |
| Death | 0 | Assumption | |
| Strategy | Cost ($) | Incremental costs ($) | Effectiveness (QALYs) | Incremental effectiveness (QALYs) | ICUR ($/QALYs) |
|---|---|---|---|---|---|
| CXR: chest X-ray; QALYs: quality-adjusted life years; ICUR: incremental cost-utility ratio; LDCT: low-dose computed tomography. | |||||
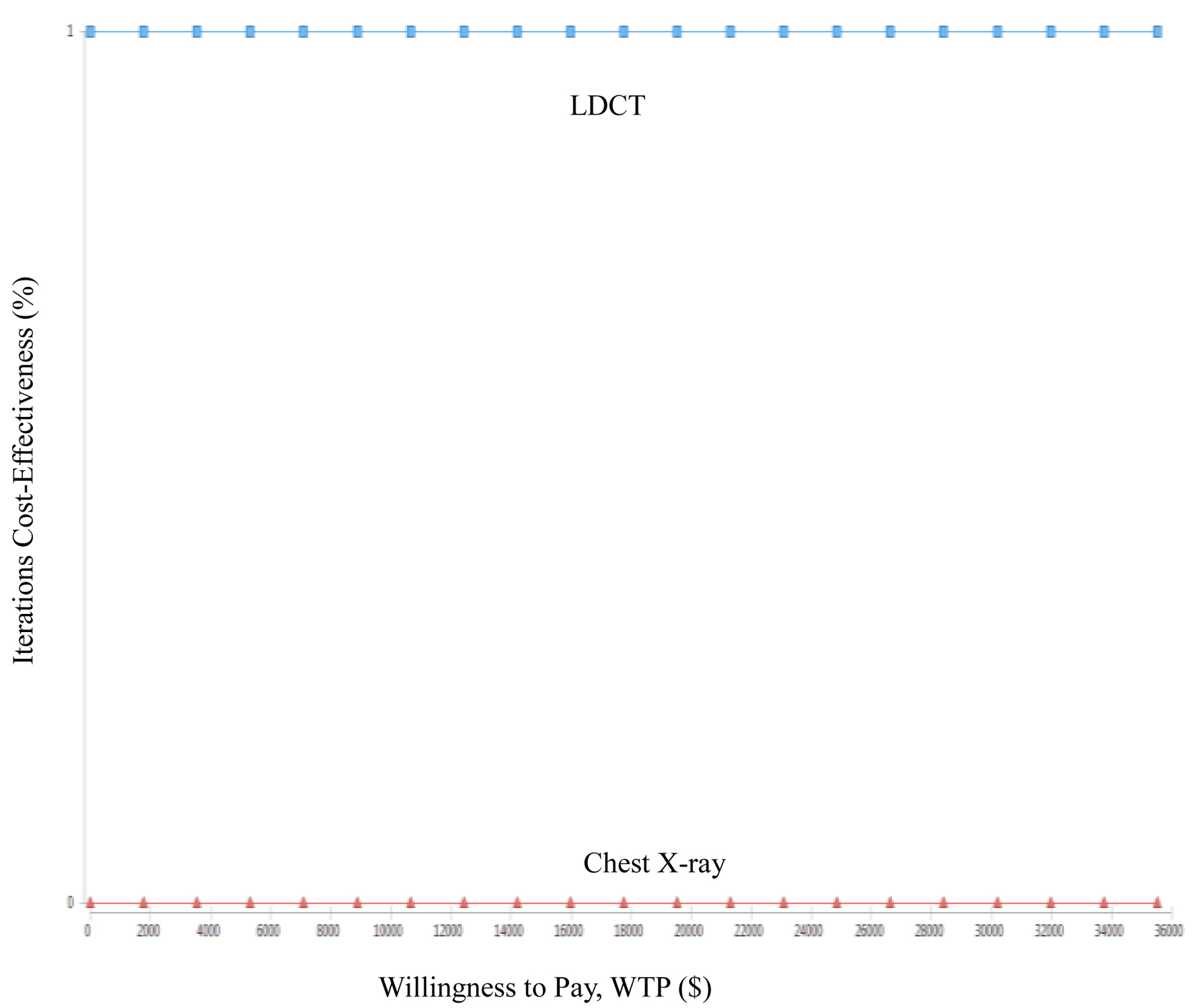
| LDCT | 84,111 | -35,436 | 3.57 | 1.43 | -24,757.65 |
| CXR | 119,567 | 2.13 | |||
| Age group | Population | Smoking rate | Population with smoking |
|---|---|---|---|
| Source: 2020 Taiwan Population Census and Smoking Prevalence Survey [3]. | |||
| 40 - 44 | 2,016,609 | 19.80% | 399,289 |
| 45 - 49 | 1,760,217 | 21.80% | 383,727 |
| 50 - 54 | 1,806,643 | 17.90% | 323,389 |
| 55 - 59 | 1,824,832 | 11.20% | 204,381 |
| 60 - 64 | 1,677,085 | 9.20% | 154,292 |
| 65 - 69 | 1,445,839 | 7.40% | 106,992 |
| 70 - 74 | 902,349 | 7.40% | 66,774 |
| 75 - 79 | 588,493 | 7.40% | 43,548 |
| 80 - 84 | 445,423 | 7.40% | 32,961 |
| 85 - 89 | 255,428 | 7.40% | 18,902 |
| 90 - 94 | 117,104 | 7.40% | 8,666 |
| 95 - 99 | 28,437 | 7.40% | 2,104 |
| 100+ | 4,242 | 7.40% | 314 |
| ≥ 40 years | 12,872,701 | 13.56% | 1,745,339 |
| Authors (years) | Country/study design | Study subjects | Perspectives | Willingness to pay | Major findings |
|---|---|---|---|---|---|
| CXR: chest X-ray; HALYs: health-adjusted life years; ICER: incremental cost-effectiveness ratio; LDCT: low-dose computed tomography; NELSON: Nederlands-Leuvens Longkanker Screenings Onderzoek; QALE: quality-adjusted life expectancy; QALYs: quality-adjusted life years. | |||||
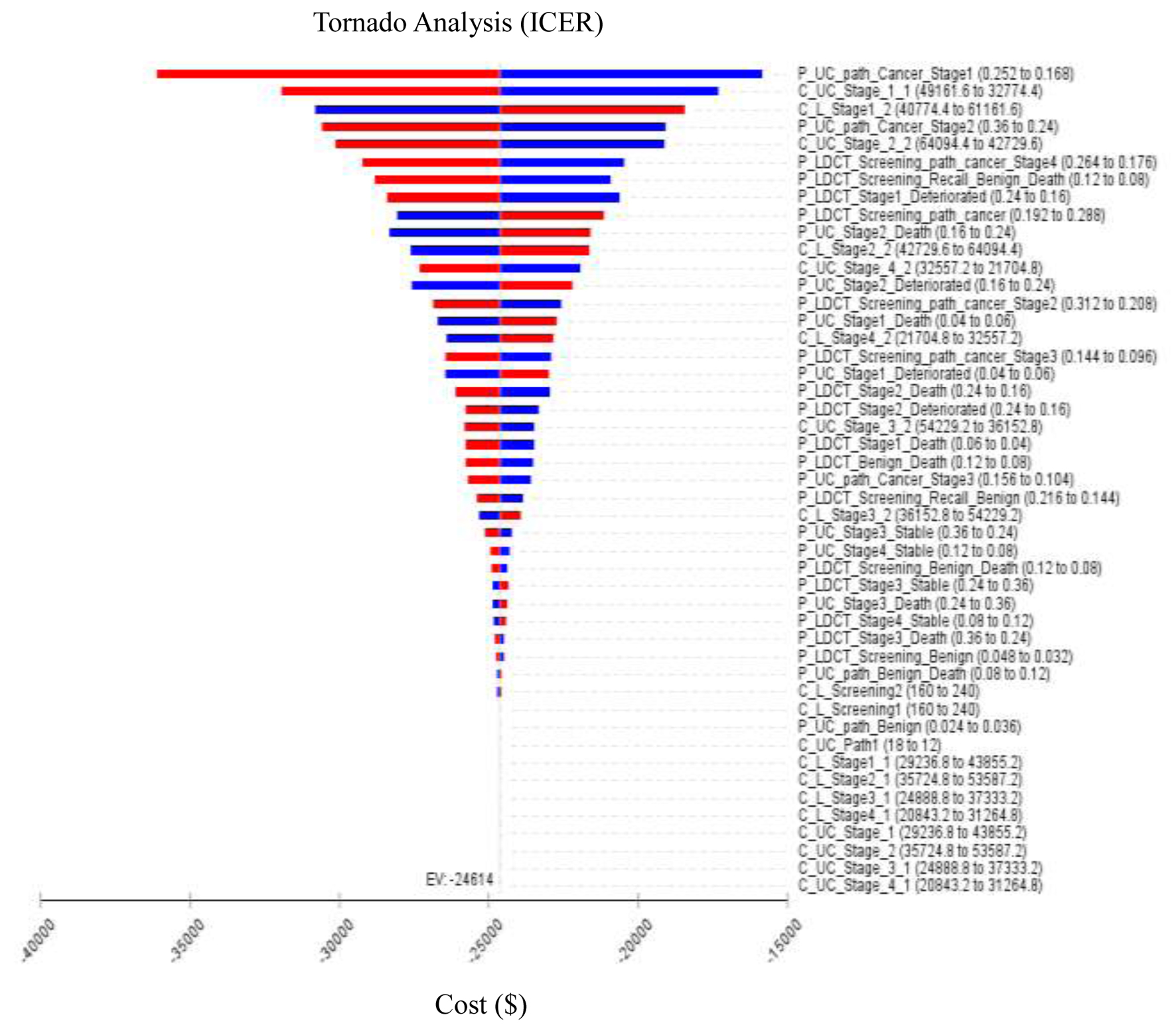
| Present study | Taiwan, Republic of China | 55 - 74 years (a high-risk population) | Health provider | $35,514 | 1. ICER of early lung cancer screening compared LDCT to CXR is US$-24,757.65/QALYs. 2. ICER is highly correlated with the recall rate and treatment costs, while the correlation with biopsy rate and examination costs is lower. 3. The probability of LDCT compared to CXR being more cost-saving is 100%. |
| Manser et al (2005) [26] | Australia | 55 - 75 years (≥ 30 packs/year smokers) | Government payer | $50,000 | 1. For male smokers aged 60 - 64 years, the ICER was $57,325/LYs and $105,090/QALYs. 2. For females aged 60 - 64 years, the ICER was $51,00/LYs and $88,583/QALYs. |
| Yang et al (2017) [17] | Taiwan, Republic of China | 55 - 75 years (≥ 30 packs/year smokers) | Government payer | $22,755 | 1. After dividing this by savings of loss-of-QALE (1.16 QALYs), the ICER was US$19,683/QALYs. 2. This ratio would fall to US$10,947/QALYs if the stage distribution for CT screening was the same as that of screen-detected cancers in the NELSON trial. |
| Tomonaga et al (2018) [27] | Switzerland | A cohort born between 1935 and 1965 | Government Payer | €50,000 | 1. The cost-effectiveness of LDCT screening for lung cancer to be better than €50,000 per LYG (or €70,000 per QALY) for all assessed screening scenarios. 2. These scenarios reduced lung cancer mortality by 6-15% while increasing incidence of lung cancer diagnoses by 2-6%. |
| McLeod et al (2020) [28] | New Zealand | 55 - 74 years (≥ 30 packs/year smokers and those who have quitting within the last 15 years | Government Payer | NZ$45,000 | LDCT screening compared to usual care in New Zealand is likely to be cost-effective for the total population: NZ$34,400/HALYs. |