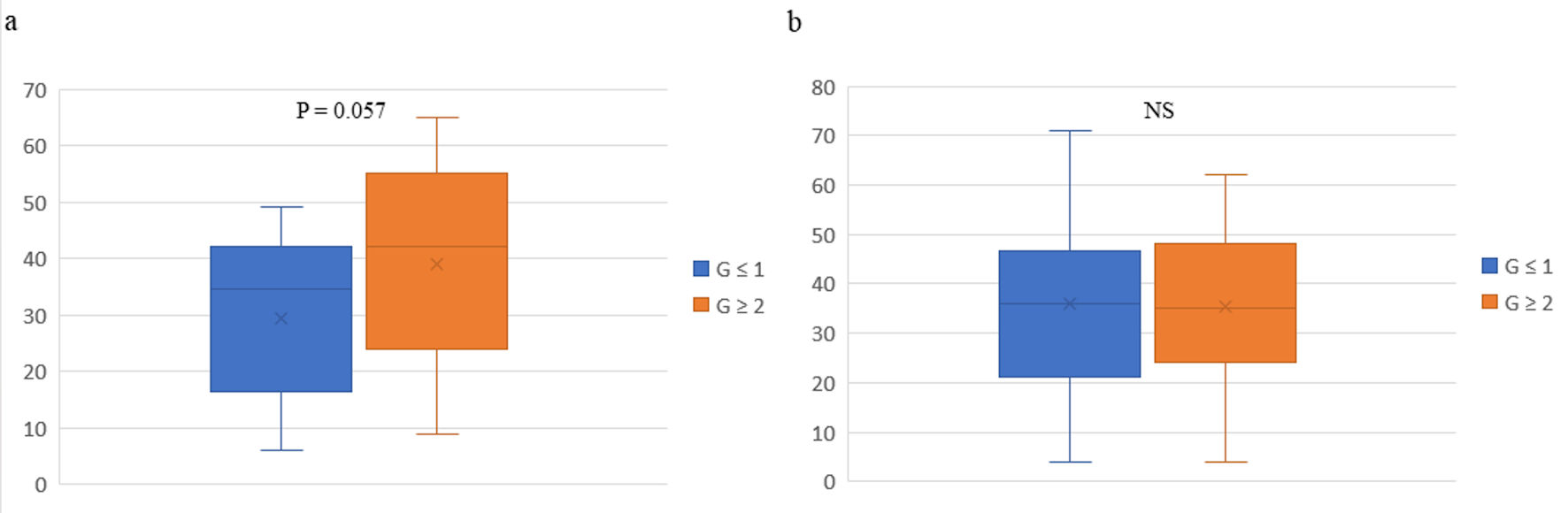
Figure 1. Box plots of pathological responses in primary tumors and peripheral natural killer cell activity before (a) and after (b) preoperative chemotherapy in 43 patients with breast cancer. The vertical axis represents peripheral natural killer cell activity. The box-plot analysis was performed using Excel software and individual data on pNK cell activity. The whiskers are error bars representing the minimum - maximum range, with the interquartile (25th - 75th percentile) range extended 1.5 times. The bottoms and tops of the boxes are the 25th and 75th percentiles, respectively, the lines in the boxes are the 50th percentiles (medians), and the Xs are the means. G: grade; NS: not significant.
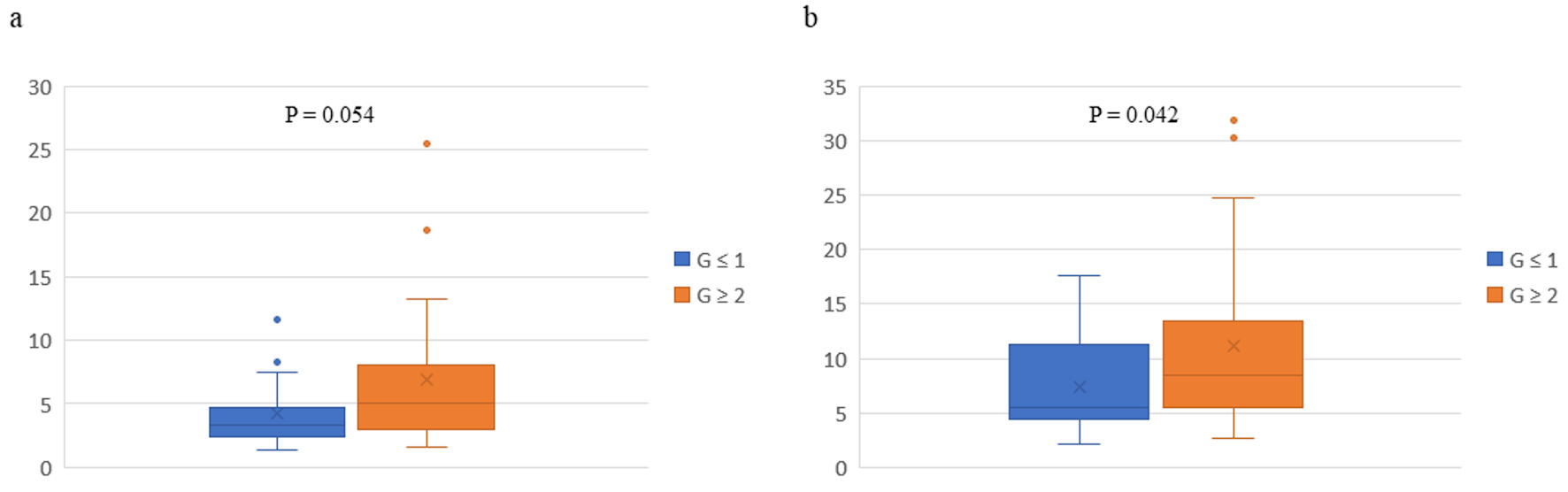
Figure 2. Box plots of pathological responses in primary tumors and percentages of CD56+ CD16- natural killer (NK) cells before (a) and after (b) preoperative chemotherapy in 43 patients with breast cancer. The vertical axis represents percentage of CD56+ CD16- NK cells. The whiskers are error bars representing the minimum - maximum range, with the interquartile (25th - 75th percentile) range extended 1.5 times. The bottoms and tops of the boxes are the 25th and 75th percentiles, respectively, the lines in the boxes are the 50th percentiles (medians), outliers are shown as closed circles, and the Xs are the means. G: grade.
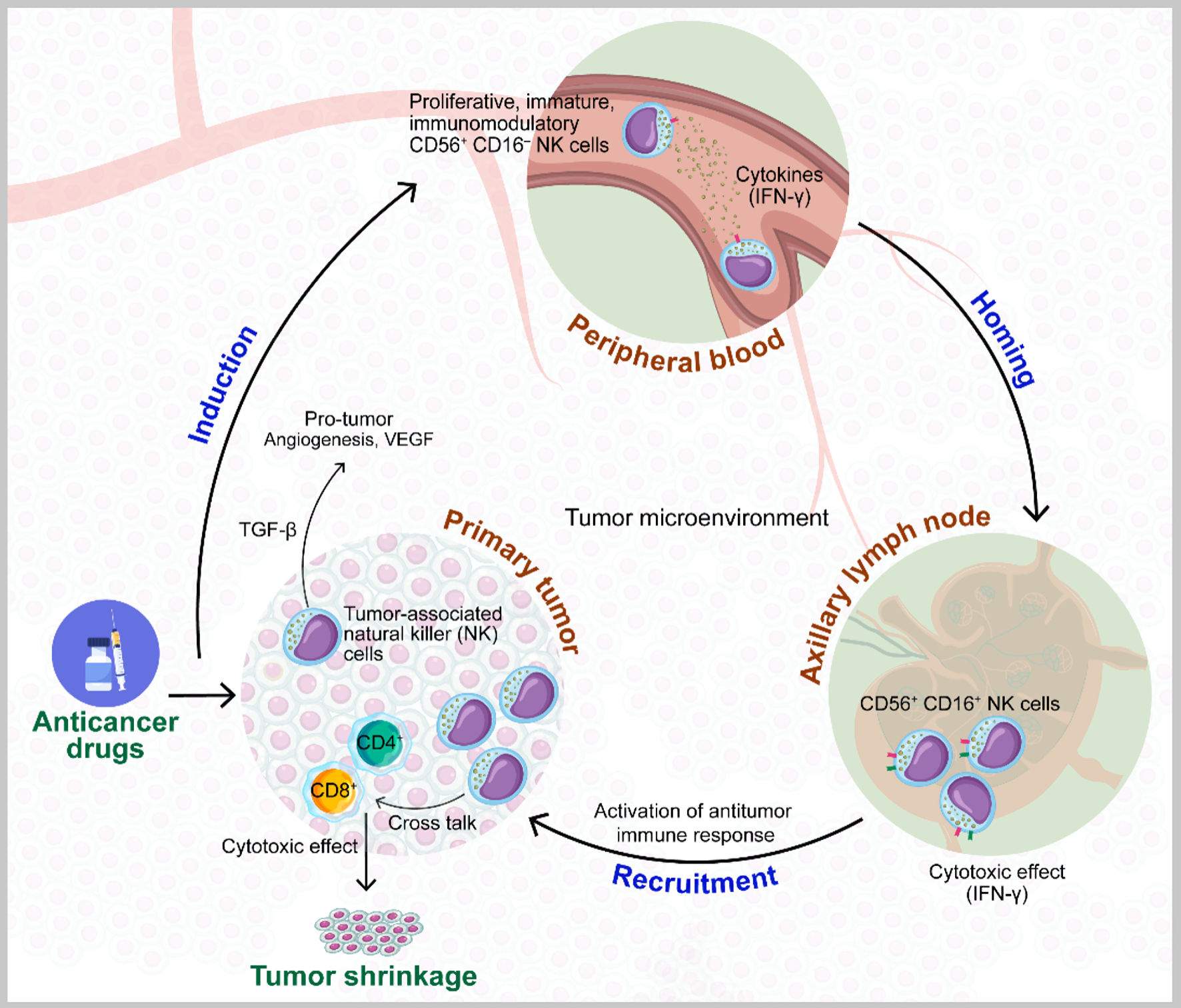
Figure 3. Model of the role of natural killer (NK) cells in the improvement of the effects of preoperative chemotherapy for breast cancer. Larger quantities of CD56+ CD16- NK cells in peripheral blood before and after chemotherapy are recruited to primary tumor sites, where they are converted from this immunomodulatory state with poor cytolytic effects to CD56+ CD16+ cells with cytotoxic effects via the production of interferon gamma (IFN-γ), which can contribute to primary tumor shrinkage in crosstalk with CD4 and CD8 T cells. Conversely, the phenotype of tumor-associated CD56+ CD16- NK cells is also involved in tumor growth and angiogenesis, derived from transforming growth factor beta (TGF-β) in the tumor microenvironment (TME). VEGF: vascular endothelial growth factor.


