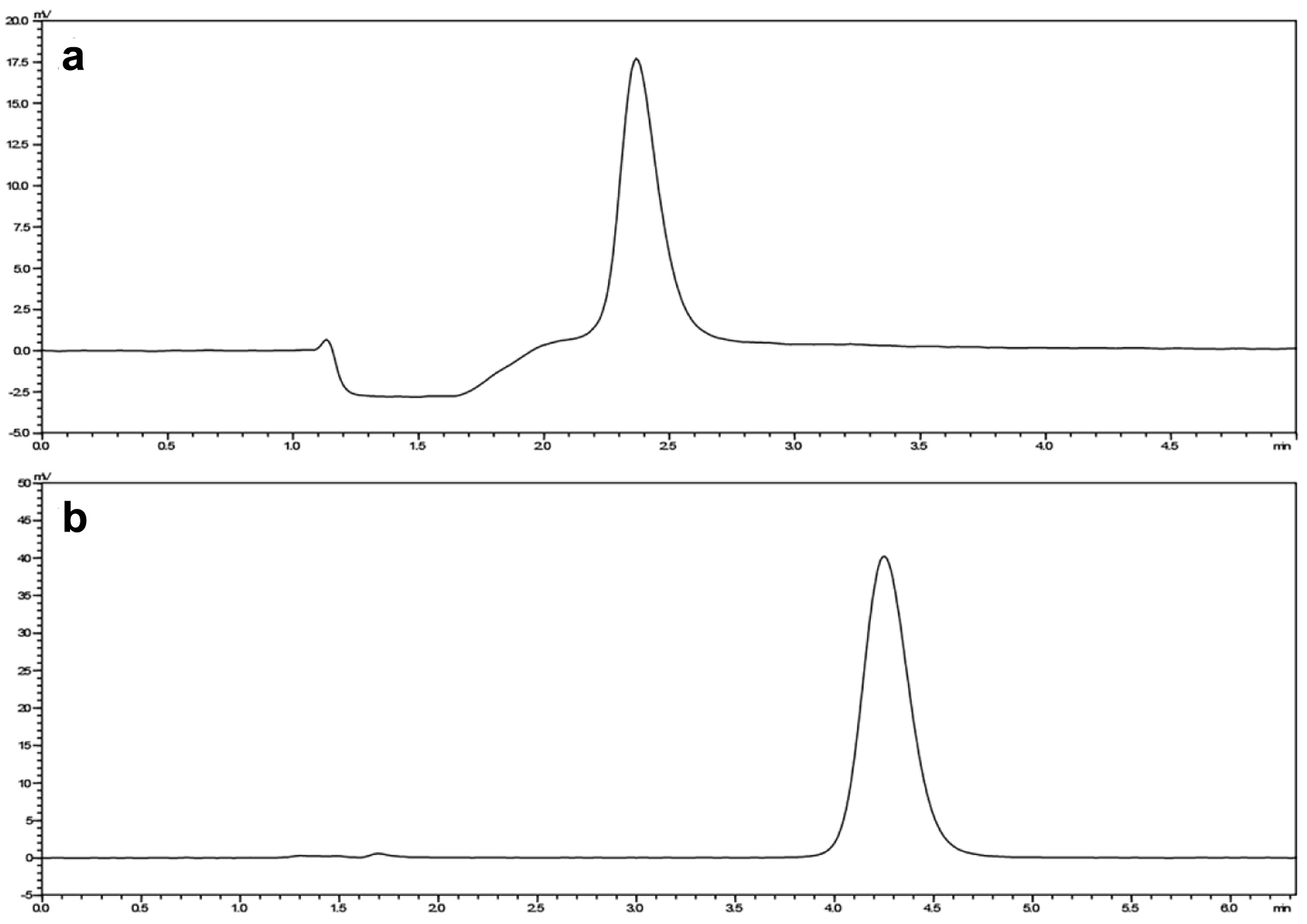
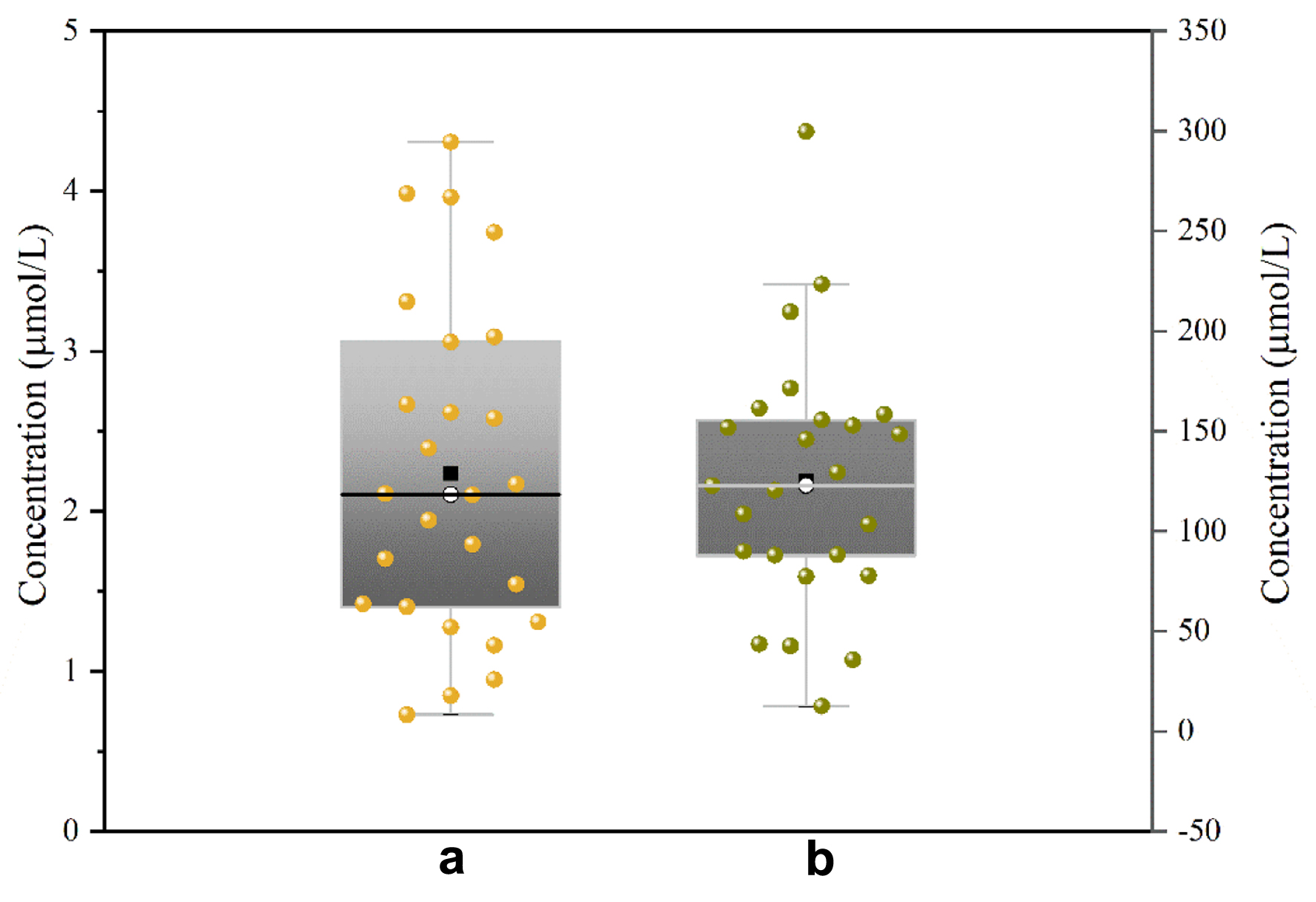
Figure 1. The peak area of MTX LC1 (a) and LC2 (b). LC1: first-dimensional liquid chromatography system; LC2: second-dimensional liquid chromatography system; MTX: methotrexate.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 5, October 2024, pages 825-836
Simultaneous Determination of Methotrexate Concentrations in Human Plasma and Cerebrospinal Fluid Using Two-Dimensional Liquid Chromatography: Applications in Primary Central Nervous System Lymphoma
Figures

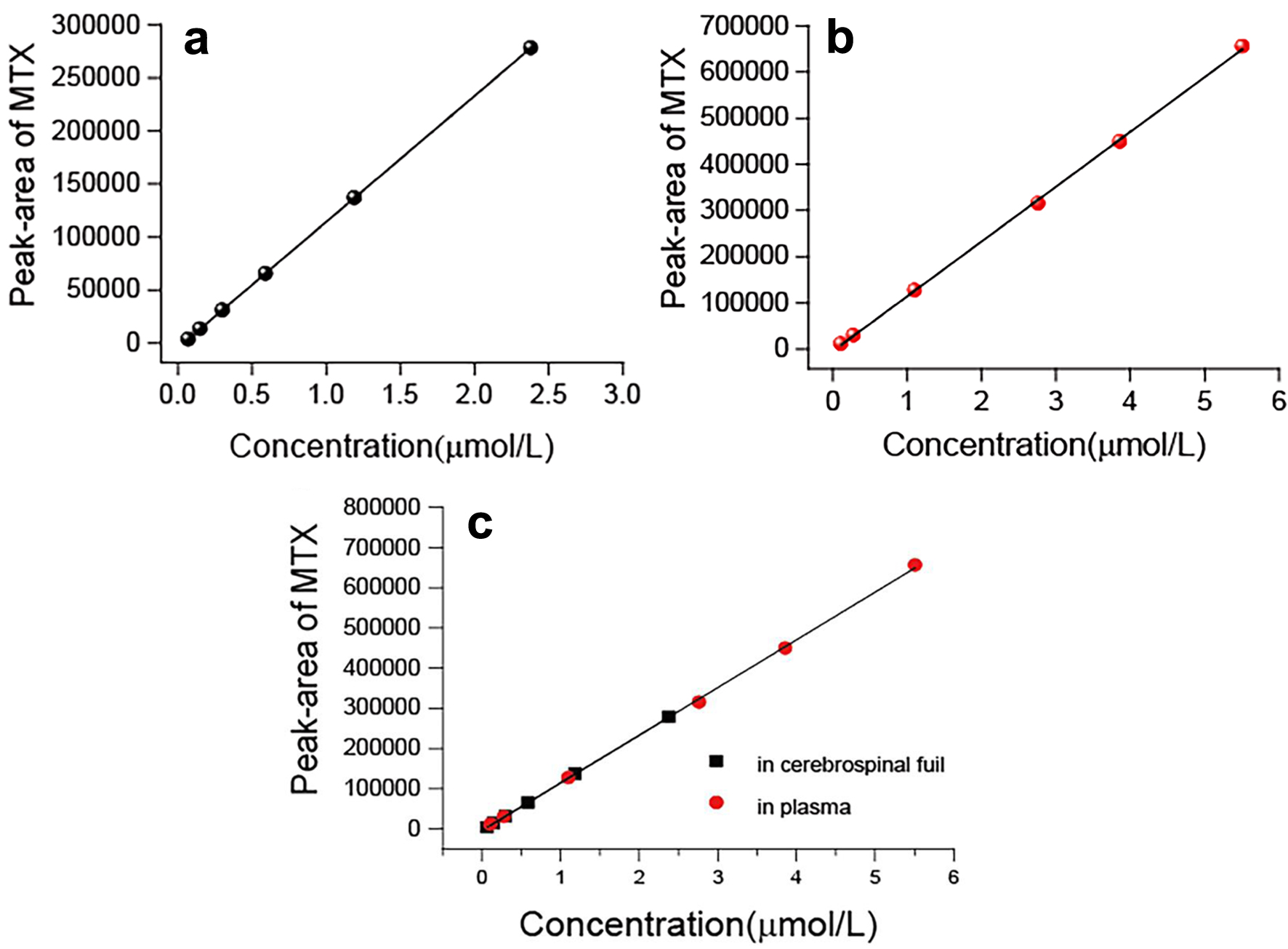
Tables
| QC levels (µmol/L) | Intra-day | Inter-day | |||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | ||
| MTX: methotrexate; QC: quality control. | |||||
| Human plasma | 0.07 | -0.46 | 4.93 | 0.26 | 0.60 |
| 0.55 | -0.33 | 1.34 | 0.33 | 3.79 | |
| 1.38 | 1.10 | 3.25 | -1.33 | 3.78 | |
| 4.13 | 0.60 | 1.93 | 0.66 | 2.31 | |
| Cerebrospinal fluid | 0.05 | 1.32 | 2.72 | 0.47 | 0.67 |
| 0.24 | 0.36 | 0.49 | 1.33 | 2.82 | |
| 0.71 | 2.17 | 4.05 | 2.30 | 3.56 | |
| 1.49 | 1.60 | 0.36 | 1.48 | 2.42 | |
| QC levels (µmol/L) | Recovery | ||
|---|---|---|---|
| Mean ± SD (%) | RSD (%) | ||
| MTX: methotrexate; QC: quality control; RSD: relative standard deviation; SD: standard deviation. | |||
| Human plasma | 0.55 | 99.33 ± 2.12 | 3.88 |
| 1.38 | 100.55 ± 2.70 | 1.95 | |
| 4.13 | 99.99 ± 2.90 | 0.7 | |
| Cerebrospinal fluid | 0.24 | 100.69 ± 4.04 | 1.67 |
| 0.71 | 100.85 ± 1.61 | 2.25 | |
| 1.49 | 99.84 ± 1.46 | 3.61 | |
| Condition | QC levels (µmol/L) | Mean ± SD (%) | RSD (%) | |
|---|---|---|---|---|
| QC: quality control; RSD: relative standard deviation; SD: standard deviation. | ||||
| Human plasma | Freeze-thaw stability (three cycles) | 0.55 | 98.12 ± 1.70 | 3.16 |
| 1.38 | 99.47 ± 1.74 | 1.27 | ||
| 4.13 | 100.89 ± 4.16 | 1 | ||
| Short-term stability (6 h at room temperature) | 0.55 | 99.76 ± 0.74 | 1.34 | |
| 1.38 | 99.04 ± 4.73 | 3.46 | ||
| 4.13 | 99.86 ± 1.55 | 0.37 | ||
| Long-term stability 30 days at -80 °C) | 0.55 | 100.54 ± 1.57 | 2.84 | |
| 1.38 | 100.74 ± 2.95 | 2.12 | ||
| 4.13 | 99.80 ± 0.35 | 0.85 | ||
| Cerebrospinal fluid | Freeze-thaw stability (three cycles) | 0.24 | 99.31 ± 0.42 | 1.75 |
| 0.71 | 100.47 ± 1.42 | 1.99 | ||
| 1.49 | 100.33 ± 0.87 | 0.66 | ||
| Short-term stability (6 h at room temperature) | 0.24 | 98.75 ± 0.85 | 3.61 | |
| 0.71 | 99.15 ± 1.85 | 2.63 | ||
| 1.49 | 100.56 ± 2.00 | 2.46 | ||
| Long-term stability (30 days at -80 °C) | 0.24 | 99.17 ± 0.30 | 1.26 | |
| 0.71 | 100.09 ± 1.17 | 1.64 | ||
| 1.49 | 100.83 ± 0.47 | 0.31 | ||
| No. | Mode of administration | Sex | Age (years) | Weight (kg) | Fluid concentration (µmol/L) | Plasma (µmol/L) | Combined with intravenous mannitol |
|---|---|---|---|---|---|---|---|
| a24 h. b48 h. c72 h. | |||||||
| 1 | 6.5 g | Male | 59 | 67.5 | 1.275 | 299.644 | No |
| 2 | 5 g | Male | 62 | 69 | 4.306 | 155.573 | No |
| 3 | 6.5 g | Male | 59 | 69 | 3.310 | 171.422 | No |
| 4 | 3.5 g | Female | 45 | 70 | 0.848 | 77.380 | Yes |
| 5 | 3.5 g | Male | 72 | 65 | 2.101 | 87.899 | No |
| 6 | 6 g | Female | 46 | 66 | 1.403 | 88.177 | Yes |
| 7 | 6 g | Male | 50 | 80 | 1.162 | 152.761 | Yes |
| 8 | 5.16 g | Male | 66 | 60 | 3.961 | 223.446 | Yes |
| 9 | 6 g | Male | 72 | 66 | 2.394 | 145.875 | No |
| 10 | 6 g | Male | 50 | 85 | 1.420 | 120.460 | Yes |
| 11 | 6 g | Female | 46 | 66 | 1.793 | 161.457 | No |
| 12 | 6.5 g | Male | 59 | 69 | 3.983 | 12.554 | Yes |
| 13 | 3.5 g | Male | 62 | 69.5 | 2.168 | 129.279 | No |
| 14 | 6 g | Male | 72 | 66 | 2.109 | 158.315 | No |
| 15 | 6 g | Male | 50 | 88 | 1.943 | 108.490 | Yes |
| 16 | 4.5 g | Female | 46 | 65 | 1.543 | 151.782 | No |
| 17 | 6 g | Male | 50 | 88 | 3.089 | 103.449 | Yes |
| 18 | 4.7 g | Female | 70 | 60 | 1.703 | 122.792 | Yes |
| 19 | 5 g | Male | 68 | 59 | 2.668 | 209.483 | No |
| 20 | 4 g | Male | 52 | 58 | 1.307 | 42.774 | Yes |
| 21 | 6 g | Male | 55 | 90 | 2.580 | 89.987 | No |
| 22 | 1.7 g | Female | 68 | 67.5 | 0.946 | 35.847 | No |
| 23 | 6 g | Male | 72 | 66 | 2.617 | 148.489 | Yes |
| 24 | 3 g | Male | 52 | 57 | 0.728 | 43.607 | No |
| 25 | 6 g | Male | 55 | 90 | 3.743 | 77.833 | Yes |
| 26 | 5 g | Male | 61 | 68 | 3.057 | 4.967a | No |
| 27 | 3 g | Female | 48 | 47 | 0.512 | 0.436a | Yes |
| 28 | 5.7 g | Female | 35 | 56 | 0.097a | 3.295a | No |
| 29 | 3.5 g | Male | 61 | 65 | 0.611 | 2.169a | No |
| 30 | 5.7 g | Female | 35 | 54 | 1.309 | 0.267b | Yes |
| 31 | 3.5 g | Male | 61 | 65 | 0.037 | 1.863a | No |
| 32 | 6.4 g | Male | 52 | 64 | 1.491 | 0.991a | Yes |
| 33 | 1.8 g | Male | 32 | 63 | 0.358 | 1.461c | No |
| 34 | 3.5 g | Female | 42 | 57 | 0.526 | 0.099c | Yes |
| 35 | 1.5 g | Female | 57 | 60 | 1.975 | 0.042c | No |