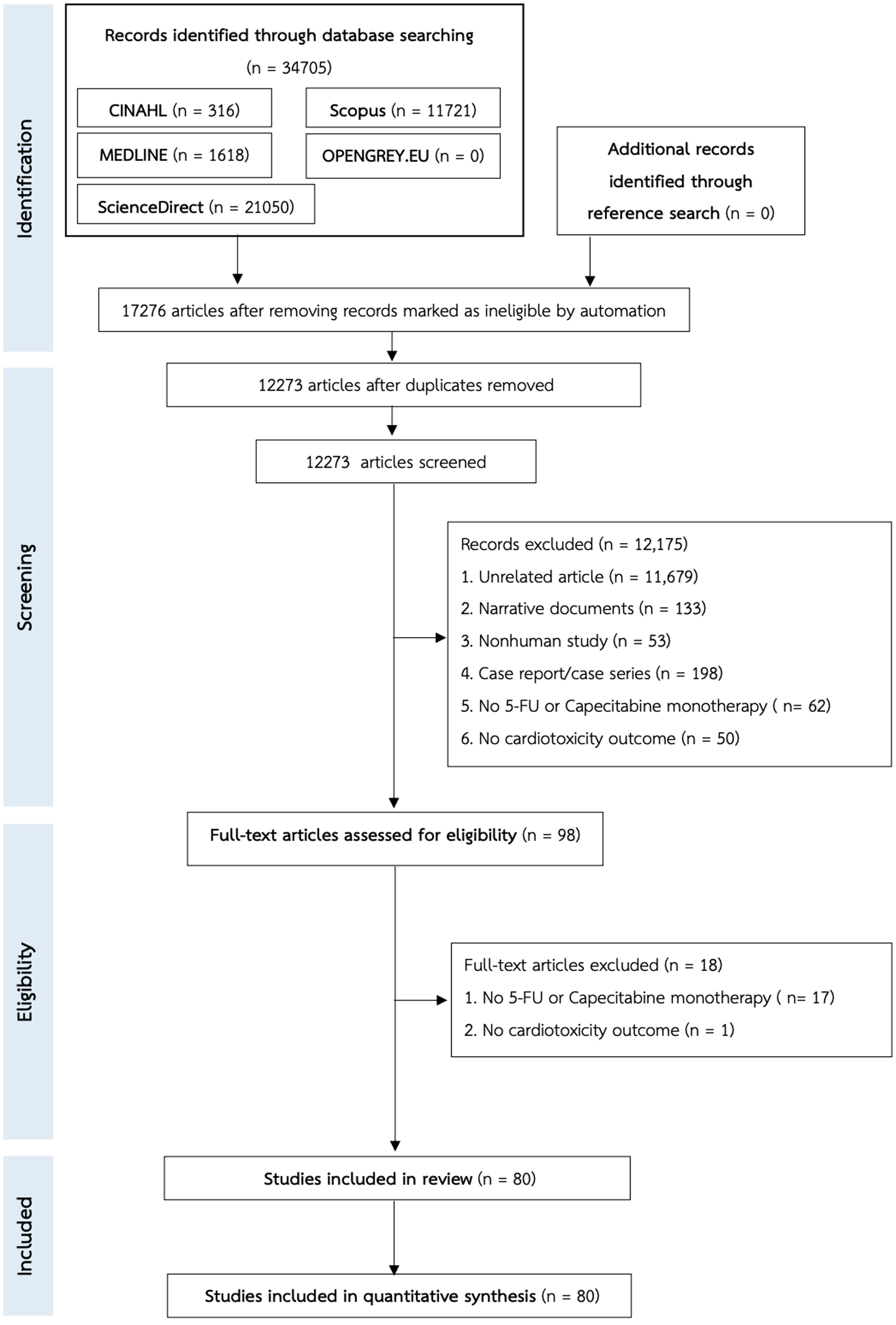
Figure 1. The PRISMA flow chart of the study selection.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 000, Number 000, October 2024, pages 000-000
The Prevalence of 5-Fluorouracil and Capecitabine Cardiotoxicity: A Systematic Review and Meta-Analysis
Figures

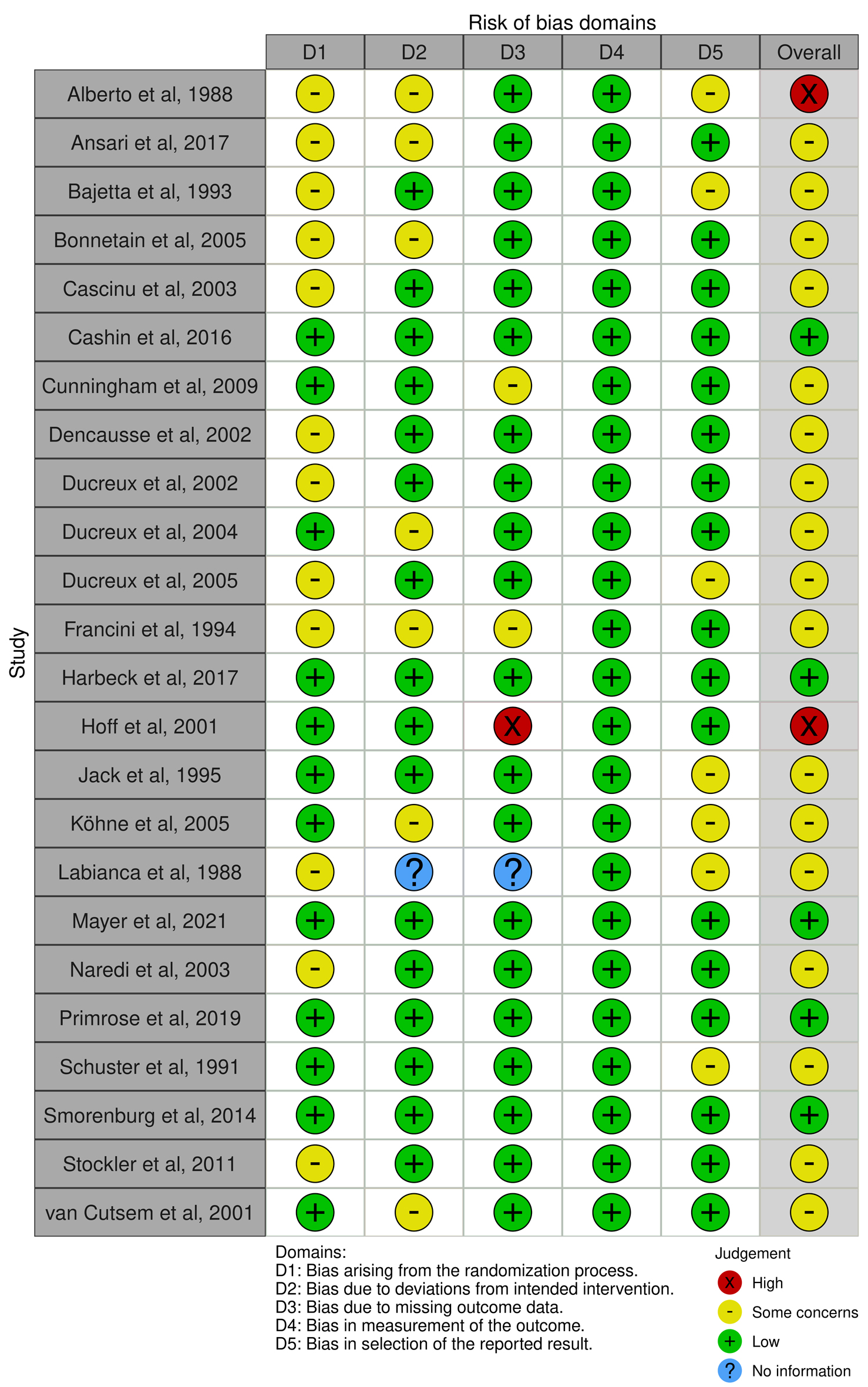
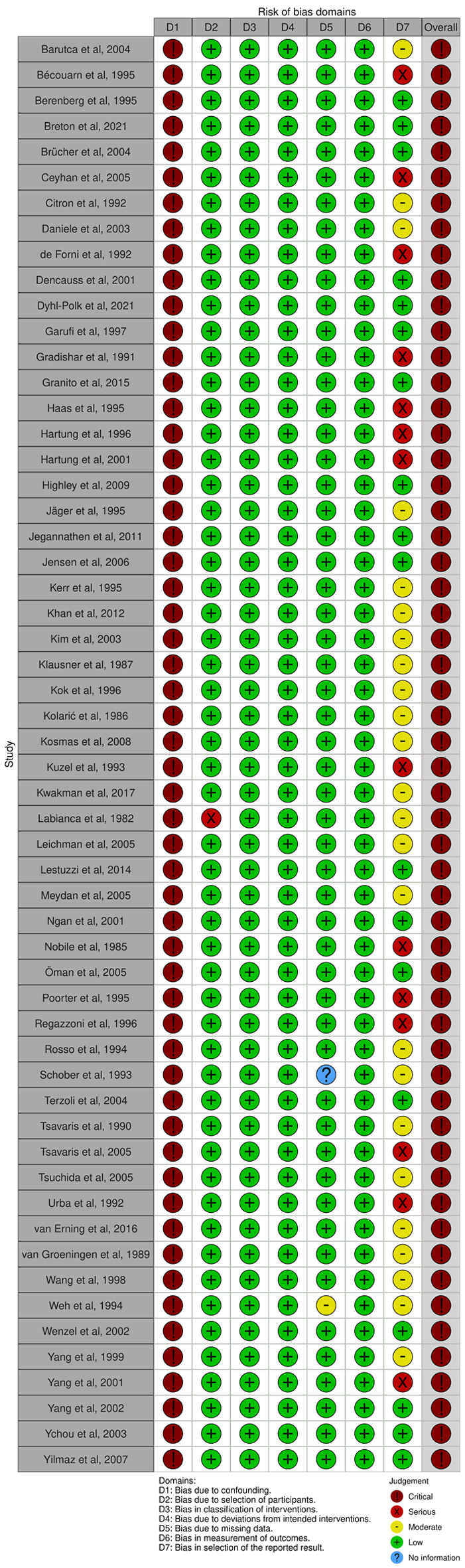
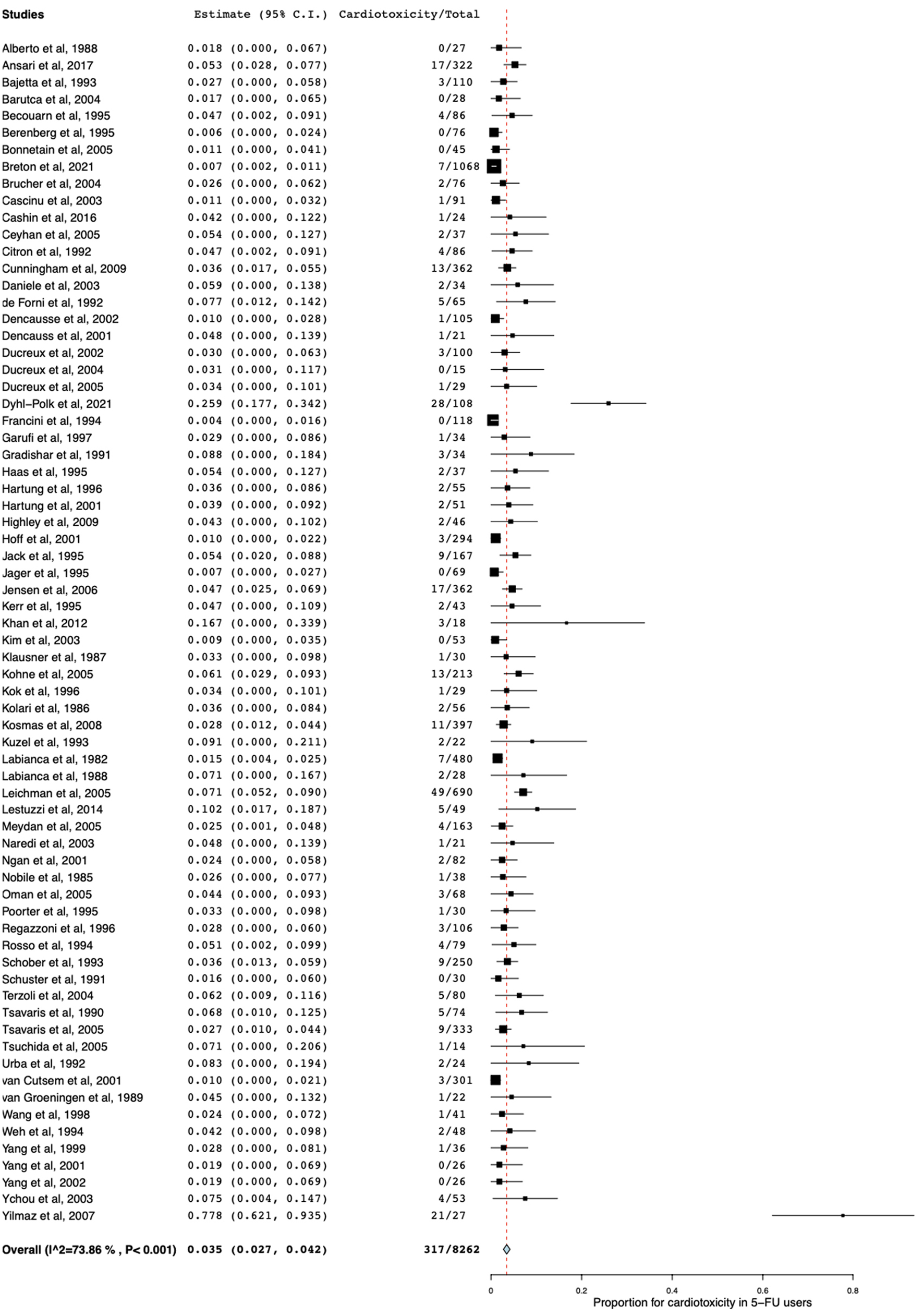
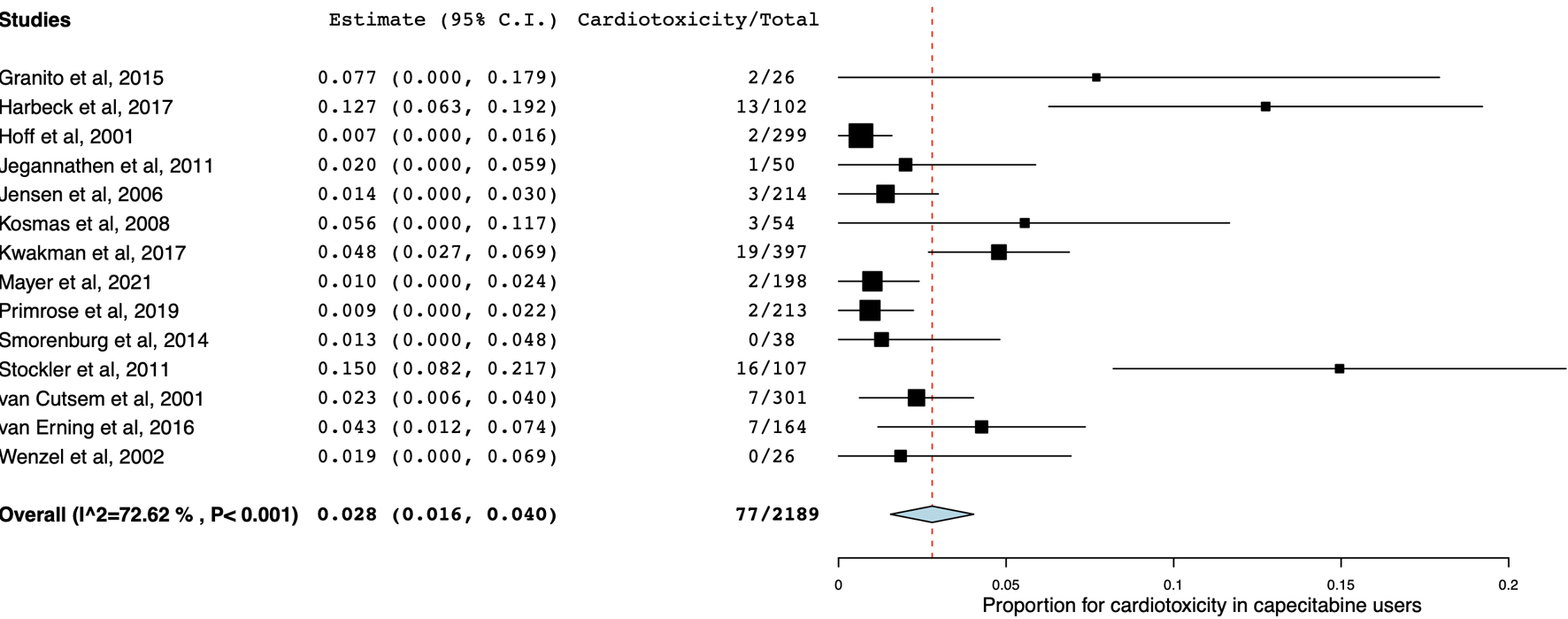
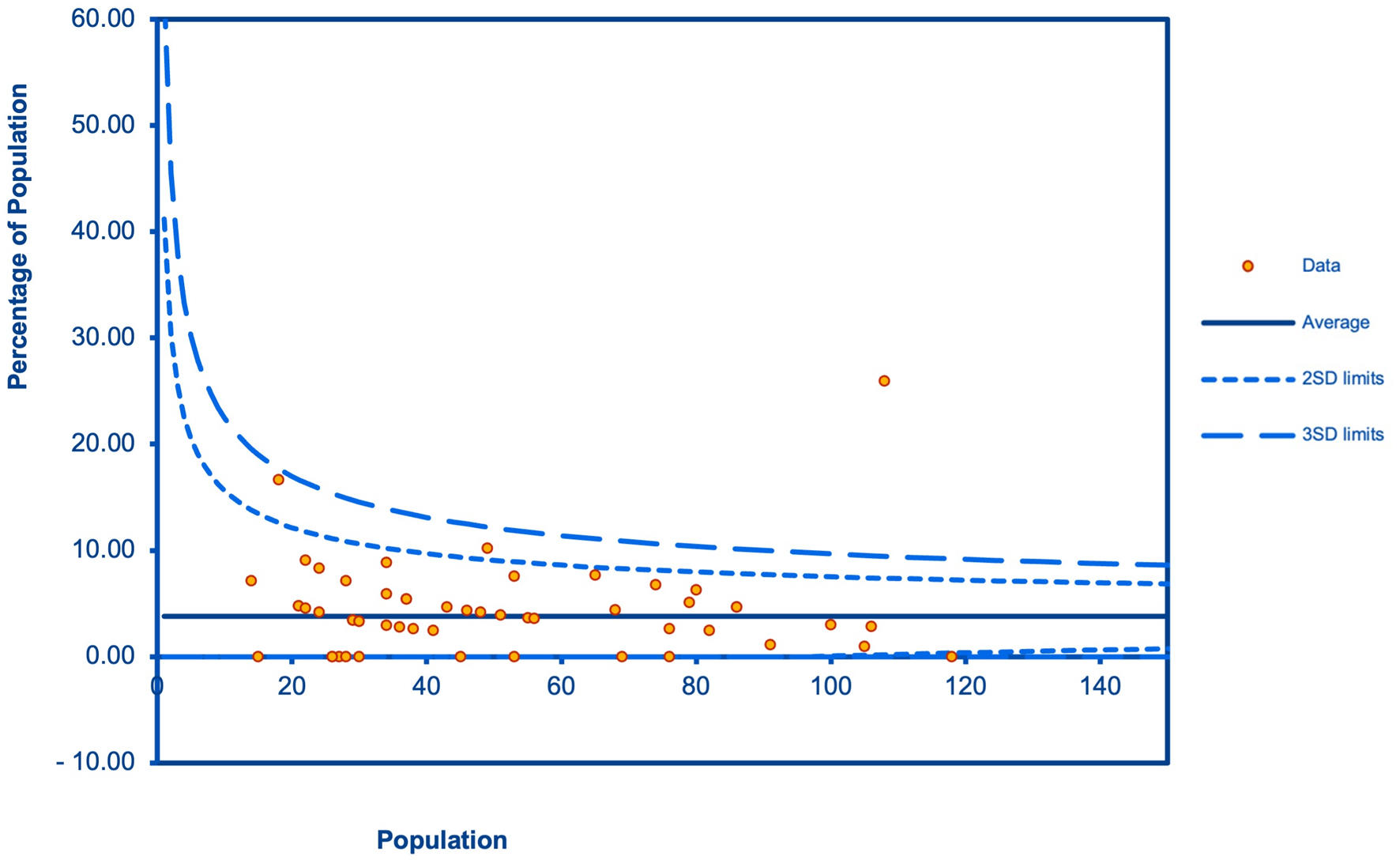
Table
| No. | Author, year | Region | Study design | Sample size | No. of participantsa | Study duration (months) | Age (years) | Male (N, %) | Comorbidity | Cancer type | Prior CMT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm A | Arm B | Arm C | |||||||||||
| aDetails of the dosage regimen for each arm are shown in Supplementary Materials 3 and 4 (www.wjon.org). 5-FU: 5-fluorouracil; CMT: chemotherapy; LV: leucovorin; N/A: not applicable; PLD: pegylated liposomal doxorubicin; RT: radiotherapy; UFT: uracil/ftorafur. | |||||||||||||
| 1 | Alberto et al, 1988 [27] | Switzerland | Randomized trial | 52 | 27 | 25 | - | 10.1 | 61.5 ± 2.12 | 27, 51.92% | N/A | Colon or rectum cancer | None |
| 2 | Ansari et al, 2017 [28] | Australia and New Zealand | Randomized controlled trial | 322 | 161 | 161 | - | 3 | 62.75 ± 10.05 | 235, 72.98% | N/A | Rectal cancer | N/A |
| 3 | Bajetta et al, 1993 [29] | Italy | Prospective randomized Trial | 222 | 110 | 112 | - | 16.75 | 61.34 ± 9.18 | 123, 55.41% | N/A | Advanced colorectal cancer | None |
| 4 | Barutca et al, 2004 [30] | Turkey | Prospective study | 28 | 28 | - | - | 15 days | 67.51 ± 10.44 | 17, 61% | N/A | Colorectal cancer, gastrointestinal system cancers | N/A |
| 5 | Becouarn et al, 1995 [31] | France | Prospective study | 86 | 86 | - | - | 52 | 61.81 ± 8.79 | 42, 48.84% | N/A | Advanced colorectal cancer | Palliative chemotherapy (8.14%) |
| 6 | Berenberg et al, 1995 [32] | United States | Phase II clinical study | 76 | 76 | - | - | 5 | N/A | N/A | N/A | Advanced gastric cancer | None |
| 7 | Bonnetain et al, 2005 [33] | France | Randomized phase II trial | 134 | 45 | 89 | - | 6 | 63.26 ± 7.73 | 110, 82.09% | N/A | Metastatic gastric cancer | N/A |
| 8 | Breton et al, 2021 [34] | France | Pooled analysis | 2,190 | 1,068 | 395 | 727 | 3.6 | 66.8 ± 2.33 | 654, 61.2% | N/A | Metastatic colorectal cancer | N/A |
| 9 | Brucher et al, 2004 [35] | Germany | Prospective study | 76 | 76 | - | - | 64.8 | 54 ± 6.48 | 59, 77.6% | N/A | Esophageal squamous cell carcinoma | N/A |
| 10 | Cascinu et al, 2003 [36] | Italy | Randomized controlled trial | 183 | 91 | 92 | - | 48 | 61.84 ± 8.58 | 100, 54.64% | N/A | Stage III colon cancer | N/A |
| 11 | Cashin et al, 2016 [37] | Sweden | Randomized controlled trial | 48 | 24 | 24 | - | 78 | 60±10.61 | 24, 50% | N/A | Colorectal peritoneal metastases | N/A |
| 12 | Ceyhan et al, 2005 [38] | Turkey | Prospective study | 37 | 37 | - | - | N/A | 60 | 26, 70.27% | N/A | Colorectal cancer, gastric cancer, breast cancer, metastatic lung carcinoid, nasopharyngeal carcinoma | N/A |
| 13 | Citron et al, 1992 [39] | United States | Prospective study | 86 | 86 | - | - | 6 | 61.26 ± 9.41 | 65, 76% | N/A | Non-small cell lung cancer | N/A |
| 14 | Cunningham et al, 2009 [40] | United Kingdom | Randomized controlled trial | 725 | 363 | 362 | - | 48 | 61.2 ± 8.89 | 126, 17.38% | N/A | Metastatic colorectal cancer | N/A |
| 15 | Daniele et al, 2003 [41] | Italy | Prospective study | 34 | 34 | - | - | 3.9 | 76.33 ± 3.59 | 23, 67.65% | Cardiovascular (55.88%), respiratory (32.35%), gastrointestinal/hepatobiliary (17.65%), genitourinary (17.65%), osteoarticular (14.71%), diabetes (17.65%), endocrinologic (5.88%) | Stage IV colorectal cancer | Previous adjuvant chemotherapy (11.76%) |
| 16 | de Forni et al, 1992 [8] | France | Prospective study | 367 | 65 | 302 | - | N/A | 55.25 ± 12.40 | 230, 62.67% | N/A | Head and neck cancer, breast cancer, colon/rectum cancer, esophagus cancer, cervix cancer | N/A |
| 17 | Dencausse et al, 2002 [42] | Germany | Prospective randomized study | 155 | 105 | 50 | - | 60 | 62.58 ± 10.03 | 108, 69.68% | N/A | Colon cancer | N/A |
| 18 | Dencausse et al, 2001 [43] | Germany | Prospective study | 21 | 21 | - | - | 6.75 | 60.69 ± 3.27 | 14, 66.67% | N/A | Rectal cancer | N/A |
| 19 | Ducreux et al, 2002 [44] | France | Randomized trial | 207 | 103 | 104 | - | 36 | 59.95 ± 9.05 | 134, 64.73% | N/A | Metastatic or locally advanced adenocarcinoma of the pancreas | None |
| 20 | Ducreux et al, 2004 [45] | France | Randomized controlled trial | 63 | 15 | 17 | 31 | N/A | 55.63 ± 11.58 | 42, 66.67% | N/A | Advanced pancreatic carcinoma | N/A |
| 21 | Ducreux et al, 2005 [46] | Belgium | Randomized phase II trial | 57 | 29 | 28 | - | 8 | 59.96 | 31, 54.39% | N/A | Locally advanced or metastatic biliary tract cancer | None |
| 22 | Dyhl-Polk et al, 2021 [47] | Denmark | Prospective study | 108 | 108 | - | - | N/A | 65.15 ± 9.11 | 59, 54.6% | Ischemic heart disease (0.9%), previous stroke (7.4%), heart failure (0.9%), atrial fibrillation (4.6%), other heart disease (2.7%), hypertension (32.4%), hypercholesterolemia (67.6%), diabetes mellitus (5.6%) | Colorectal or anal cancer | N/A |
| 23 | Francini et al, 1994 [48] | Italy | Randomized controlled trial | 239 | 118 | 121 | - | 54 | 56.76 | 126, 52.72% | N/A | Surgically resected colon cancer | N/A |
| 24 | Garufi et al, 1997 [49] | Italy | Phase I study | 34 | 34 | - | - | 9 | 55.34 ± 12.19 | 19, 55.88% | N/A | Metastatic adenocarcinoma of the colon or rectum | Prior chemotherapy (13/34) |
| 25 | Gradishar et al, 1991 [50] | United States | Retrospective review | 244 | 34 | 210 | - | N/A | N/A | N/A | N/A | Gastric cancer, Head and neck cancer | N/A |
| 26 | Granito et al, 2015 [51] | Italy | Retrospective study | 26 | 26 | - | - | 31 | 65.48 ± 7.07 | 23, 88.46% | Mild ascites (30.76%), absent ascites (69.23%) | Hepatocellular carcinoma | N/A |
| 27 | Haas et al, 1995 [52] | USA | Phase II study | 37 | 37 | - | - | N/A | 61.24 ± 9.19 | 24, 64.86% | One patient had a myocardial infarction four years before presenting colon cancer. He was maintained on stable doses of nitrates and a calcium channel blocker. | Metastatic adenocarcinoma of the colon or rectum | Adjuvant CMT 2, adjuvant CMT/RT 1, adjuvant immunotherapy/CMT 2, advanced CMT 2, immunotherapy 3, immunotherapy/RT 1, RT 5, none 22 |
| 28 | Harbeck et al, 2017 [53] | Germany | Randomized controlled trial | 210 | 105 | 105 | - | N/A | 61.82 ± 10.96 | 0 | N/A | Metastatic breast cancer | Prior PLD (37%), capecitabine (36%) |
| 29 | Hartung et al, 1996 [54] | Germany | Retrospective study | 92 | 55 | 37 | 13 | Median 59.7 | 55, 59.78% | N/A | Colon cancer, rectal cancer | N/A | |
| 30 | Hartung et al, 2001 [55] | Germany | Phase II clinical study | 51 | 51 | - | - | 20.2 | 58.35 ± 11.77 | 38, 74.51% | N/A | Metastatic colorectal cancer | N/A |
| 31 | Highley et al, 2009 [56] | United Kingdom | Phase II study | 46 | 46 | - | - | 6 | 68.09 ± 2.49 | 33, 71.74% | N/A | Transitional cell carcinoma of the urinary tract | None |
| 32 | Hoff et al, 2001 [57] | United States, Canada, Brazil, Mexico | Phase III randomized controlled study | 605 | 303 | 302 | - | 13.3 | 63.05 ± 10.98 | 378, 62.48% | N/A | Advanced or metastatic colorectal cancer | Adjuvant 5-FU (36.3%) |
| 33 | Jack et al, 1995 [58] | Southeast Scotland | Randomized controlled trial | 332 | 167 | 165 | - | Median follow-up of 15 years | 53.97 ± 8.34 | 0 | N/A | Breast cancer | N/A |
| 34 | Jager et al, 1995 [59] | Germany | Prospective study | 69 | 69 | - | - | N/A | 55.98 ± 8.67 | 50, 72.46% | N/A | Advanced colorectal and rectal carcinoma | N/A |
| 35 | Jegannathen et al, 2011 [60] | United Kingdom | Phase II clinical study | 50 | 50 | - | - | N/A | 55.35 ± 8.47 | 40, 80% | N/A | Head and neck cancer | N/A |
| 36 | Jensen et al, 2006 [25] | Denmark | Prospective study | 668 | 362 | 92 | 214 | N/A | N/A | N/A | Hypercholesterolemia, diabetes, hypertension, cerebral ischemia | Colorectal cancer, gastric cancer | N/A |
| 37 | Kerr et al, 1995 [61] | United Kingdom | Phase I clinical trial | 43 | 43 | - | - | N/A | 56 ± 9.38 | 28, 65.12% | N/A | Colorectal cancer | N/A |
| 38 | Khan et al, 2012 [62] | Pakistan | Retrospective study | 301 | 18 | 283 | - | N/A | 47.13 ± 10.99 | 75, 24.92% | N/A | N/A | N/A |
| 39 | Kim et al, 2003 [63] | Korea | Prospective study | 122 | 53 | 69 | - | N/A | 55.98 ± 11.16 | 70, 57.38% | N/A | Adenocarcinoma of the colon (colon cancer) | N/A |
| 40 | Klausner et al, 1987 [64] | Israel | Prospective study | 30 | 30 | - | - | 19 | 51.03 ± 11.03 | 20, 66.67% | N/A | Metastatic malignant melanoma | None |
| 41 | Kohne et al, 2005 [65] | Europe | Phase III prospective, multicenter, randomized, non-blinded | 427 | 213 | 214 | - | 27.6 | 60.25 ± 9.24 | 268, 62.32% | N/A | Metastatic colorectal cancer | N/A |
| 42 | Kok et al, 1996 [66] | The Netherlands | Prospective study | 29 | 29 | - | - | 24 weeks or until progression. | 59.39 ± 8.14 | 25, 86.21% | N/A | Metastatic adenocarcinoma of the esophagus or esophagogastric junction area. | None |
| 43 | Kolaric et al, 1986 [67] | Slovenia | Controlled phase III clinical study | 115 | 56 | 59 | - | N/A | 51.89 ± 8.34 | 71, 61.74% | N/A | Gastric cancer, rectosigmoid cancer | N/A |
| 44 | Kosmas et al, 2008 [68] | Greece | Prospective study | 644 | 397 | 193 | 54 | N/A | 65.91 ± 2.26 | N/A | Hyperlipidemia, obesity, chronic obstructive pulmonary disease | Colorectal cancer, head and neck cancer, breast cancer | N/A |
| 45 | Kuzel et al, 1993 [69] | USA | Phase II study | 22 | 22 | - | - | 1 | 68.29 ± 6.55 | 22, 100% | N/A | Metastatic prostate carcinomas refractory to hormonal therapy | None |
| 46 | Kwakman et al, 2017 [70] | United Kingdom | Retrospective study | 2,461*event | 397 | 2,064 | - | N/A | N/A | N/A | N/A | Colorectal cancer | N/A |
| 47 | Labianca et al, 1982 [26] | Italy | Retrospective study | 1,083 | 480 | 603 | - | N/A | N/A | N/A | Ischemic heart disease | Gastric cancer, breast cancer | N/A |
| 48 | Labianca et al, 1988 [71] | Italy | Randomized trial | 54 | 28 | 26 | - | 22 | 55.48 ± 9.53 | 33, 61% | N/A | Advanced colorectal cancer | None |
| 49 | Leichman et al, 2005 [72] | United States | Retrospective study | 690 | 340 | 350 | - | 13 | 60.78 ± 11.38 | 407, 57% | N/A | Metastatic or recurrent colorectal cancer | Previous adjuvant CMT or immunotherapy (or both) was allowed as long as ≥ 1 year had elapsed since discontinuation of therapy. No previous chemotherapy for advanced disease was permitted. |
| 50 | Lestuzzi et al, 2014 [15] | Italy and Germany | Prospective study | 231 | 49 | 182 | - | 9 | 57.5 | 148, 64.07% | Obesity (9.09%), diabetes mellitus (12.12%), hypertension (33.33%), dyslipidemia (23.81%), coronary artery disease (3.46%), active smoker (40.69%), former smoker (23.38%) | Colorectal cancer, breast cancer, head and neck cancer, gastric or bowel cancer | N/A |
| 51 | Mayer et al, 2021 [73] | Unites States | Phase III randomized controlled study | 308 | 160 | 148 | - | 58 | 51.85 ± 9.00 | 0 | N/A | Breast cancer | Prior neoadjuvant taxane 160/160, prior RT 122/160, prior neoadjuvant anthracycline 136/160 |
| 52 | Meydan et al, 2005 [74] | Turkey | Prospective study | 231 | 163 | 68 | - | N/A | 57 ± 18.47 | 138, 59.74% | Coronary artery disease, hypertension, diabetes mellitus | Colorectal cancer, gastric cancer, pancreas and gallbladder, breast cancer, neuroendocrine, head and neck cancer | N/A |
| 53 | Naredi et al, 2003 [75] | Sweden | Prospective randomized study | 39 | 21 | 18 | - | 46 | 64.38 ± 8.74 | 27, 69.23% | N/A | Metastasis colorectal cancer | N/A |
| 54 | Ngan et al, 2001 [76] | Australia and New Zealand | Prospective study | 82 | 82 | - | - | 12 | 58.74 ± 12.36 | 55, 67.07% | N/A | Localized adenocarcinoma of the rectum | N/A |
| 55 | Nobile et al, 1985 [77] | Italy | Phase II clinical study | 38 | 38 | - | - | N/A | 60.59 ± 8.43 | 20, 52.63% | N/A | Advanced colorectal cancer | 7/38 had failed prior 5-FU treatment. |
| 56 | Oman et al, 2005 [78] | Sweden | Phase I/II clinical trial | 68 | 68 | - | - | 3.9 | 62.07 ± 11.23 | 29, 42.65 % | N/A | Non-resectable pancreas cancer | N/A |
| 57 | Poorter et al, 1995 [79] | The Netherlands | Prospective study | 30 | 30 | - | - | 22 | 54.69 ± 10.54 | 12, 40% | N/A | Metastatic gastrointestinal cancer | N/A |
| 58 | Primrose et al, 2019 [80] | United Kingdom | Randomized controlled trial | 447 | 223 | 224 | - | N/A | 62.92 ± 2.48 | 224, 50.11% | N/A | Biliary tract cancer | N/A |
| 59 | Regazzoni et al, 1996 [81] | Switzerland | Retrospective study | 106 | 106 | - | - | N/A | 56 ± 10.32 | N/A | N/A | Breast cancer | 81% had previously received anthracyclines |
| 60 | Rosso et al, 1994 [82] | Italy | Prospective study | 79 | 79 | - | - | 28.75 | 61.34 ± 7.46 | 50, 63.29% | N/A | Advanced colorectal carcinoma | N/A |
| 61 | Schober et al, 1993 [83] | Germany | Prospective study | 390 | 250 | 89 | 51 | N/A | 52 ± 13.85 | 260, 66.67% | Hypertension, diabetes, hyperlipidemia, history of coronary or peripheral artery disease | Gastric cancer, colorectal cancer | Pretreatment with etoposide, adriamycin, and cisplatin 1/390 |
| 62 | Schuster et al, 1991 [84] | Germany | Randomized controlled trial | 61 | 30 | 31 | - | 24 | 56.29 ± 9.29 | 39, 63.93% | N/A | Advanced colorectal carcinoma | None |
| 63 | Smorenburg et al, 2014 [85] | The Netherland | Randomized controlled trial | 78 | 38 | 40 | - | 39 | 75.07 ± 4.36 | 0 | N/A | Metastatic breast cancer | Previous adjuvant CMT with anthracyclines was allowed, considering a cumulative dose of < 240 mg/m2 of doxorubicin or < 450 mg/m2 of epirubicin and completion for at least 12 months |
| 64 | Stockler et al, 2011 [86] | Australia, New Zealand | Randomized controlled study | 323 | 214 | 109 | - | 39.6 | 59.67 | 0 | N/A | Advanced breast cancer | N/A |
| 65 | Terzoli et al, 2004 [87] | Italy | Prospective study | 80 | 80 | - | - | 14 | 60.24 ± 9.34 | 46, 57.50% | N/A | Advanced colorectal cancer | Adjuvant CMT 13/80 |
| 66 | Tsavaris et al, 1990 [88] | Greece | Prospective study | 74 | 74 | - | - | 7.4 | 61 | 46, 62.16% | N/A | Advanced colorectal cancer | RT 16/74, 5-FU with or without mitomycin C 17/74 |
| 67 | Tsavaris et al, 2005 [89] | Greece | Prospective study | 522 | 333 | 189 | - | N/A | 62.04 ± 2.31 | N/A | Hyperlipidemia, obesity, chronic obstructive pulmonary disease | Head and neck cancer, colorectal cancer | None |
| 68 | Tsuchida et al, 2005 [90] | Japan | Retrospective study | 14 | 14 | - | - | 27 | 64.11 ± 9.08 | 12, 86% | N/A | Recurrence of esophageal squamous cell carcinoma | Cisplatin (50 - 80 mg/m2) for 1 day and 5-FU (500 - 800 mg/m2) for 5 days (eight patients) |
| 69 | Urba et al, 1992 [91] | United states | Prospective study | 24 | 24 | - | - | 12.5 | 61.04 ± 9.25 | 20, 83% | One patient died of a myocardial infarction with the risk factors of mild hypertension and mild obesity. | Resectable adenocarcinoma of the esophagus | None |
| 70 | Van Cutsem et al, 2001 [92] | Europe, Australia, New Zealand, Taiwan and Israel | Phase III randomized controlled study | 602 | 301 | 301 | - | N/A | 63.60 ± 9.59 | 343, 56.98% | N/A | Colorectal cancer | Capecitabine 56/301, 5-FU 41/301 |
| 71 | Van Erning et al, 2016 [93] | The Netherland | Retrospective study | 357 | 164 | 193 | - | N/A | 74.53 | 109, 58.60% | None | Stage III colon cancer | N/A |
| 72 | Van Groeningen et al, 1989 [94] | The Netherlands | Prospective study | 22 | 22 | - | - | 6 | 59.46 ± 12.57 | 8, 36.36% | N/A | Advanced colorectal cancer | Prior hepatic intra-arterial 3/22, IV 5-aza-2’-deoxycytidine 1/22, IV cisplatin and hepatic intra-arterial 5-FU 1/22 |
| 73 | Wang et al, 1998 [95] | Taiwan | Prospective study | 41 | 41 | - | - | 18.4 | 59.92 ± 7.62 | 33, 80.48% | N/A | Advanced colorectal cancer | N/A |
| 74 | Weh et al, 1994 [96] | Germany | Prospective study | 57 | 57 | - | - | 41 | 56.27 ± 9.38 | 36, 63.16% | N/A | Metastatic colorectal carcinoma | N/A |
| 75 | Wenzel et al, 2002 [97] | Austria | Prospective study | 26 | 26 | N/A | - | 25 | 58.9 ± 7.32 | 19, 73.08% | N/A | Metastatic renal cell carcinoma | N/A |
| 76 | Yang et al, 1999 [98] | Taiwan | Prospective study | 36 | 36 | - | - | 9 | 57.68 ± 10.64 | 21, 58.33% | N/A | Colorectal cancer | 5-FU/levamisole (4/36), 5-FU/LV (17/36) |
| 77 | Yang et al, 2001 [99] | Taiwan | Phase II clinical study | 26 | 26 | - | - | N/A | 55.48 ± 12.62 | 18, 69.23% | N/A | Advanced colorectal cancer | None |
| 78 | Yang et al, 2002 [100] | Taiwan | Prospective study | 51 | 26 | 25 | - | N/A | 59 ± 10.22 | 29, 56.9% | N/A | Metastatic colorectal cancer | Oral UFT 300 mg/m2/day plus LV 90 mg/day |
| 79 | Ychou et al, 2003 [101] | France | Prospective study | 53 | 53 | - | - | 38 | 65.05 ± 9.05 | 25, 47.17% | N/A | Metastatic colorectal cancer | Prior CMT 16/53, 30.19% |
| 80 | Yilmaz et al, 2007 [102] | Turkey | Prospective study | 27 | 27 | - | - | 24 h | 51.60 ± 12.77 | 15, 55.56% | Diabetes, hypertension | Colorectal cancer, gastric cancer, liver cancer, distal esophagus cancer | N/A |