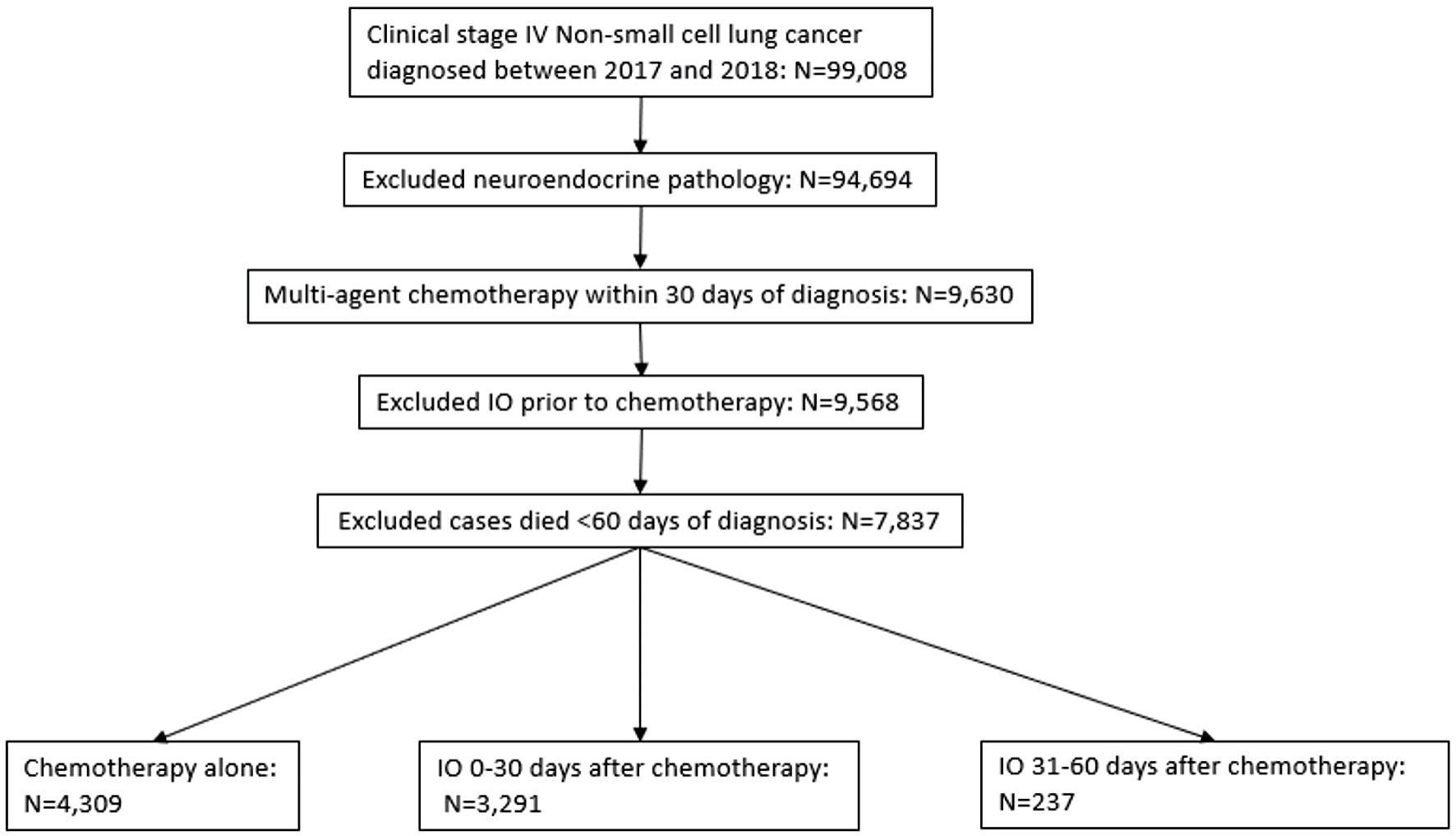
Figure 1. Selection criteria according to CONSORT diagram. Deidentified cases were released from the National Cancer Database. IO: immunotherapy.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 5, October 2024, pages 769-776
Prognostic Implications of Timing of Immunotherapy in Stage IV Non-Small Cell Lung Cancer
Figures

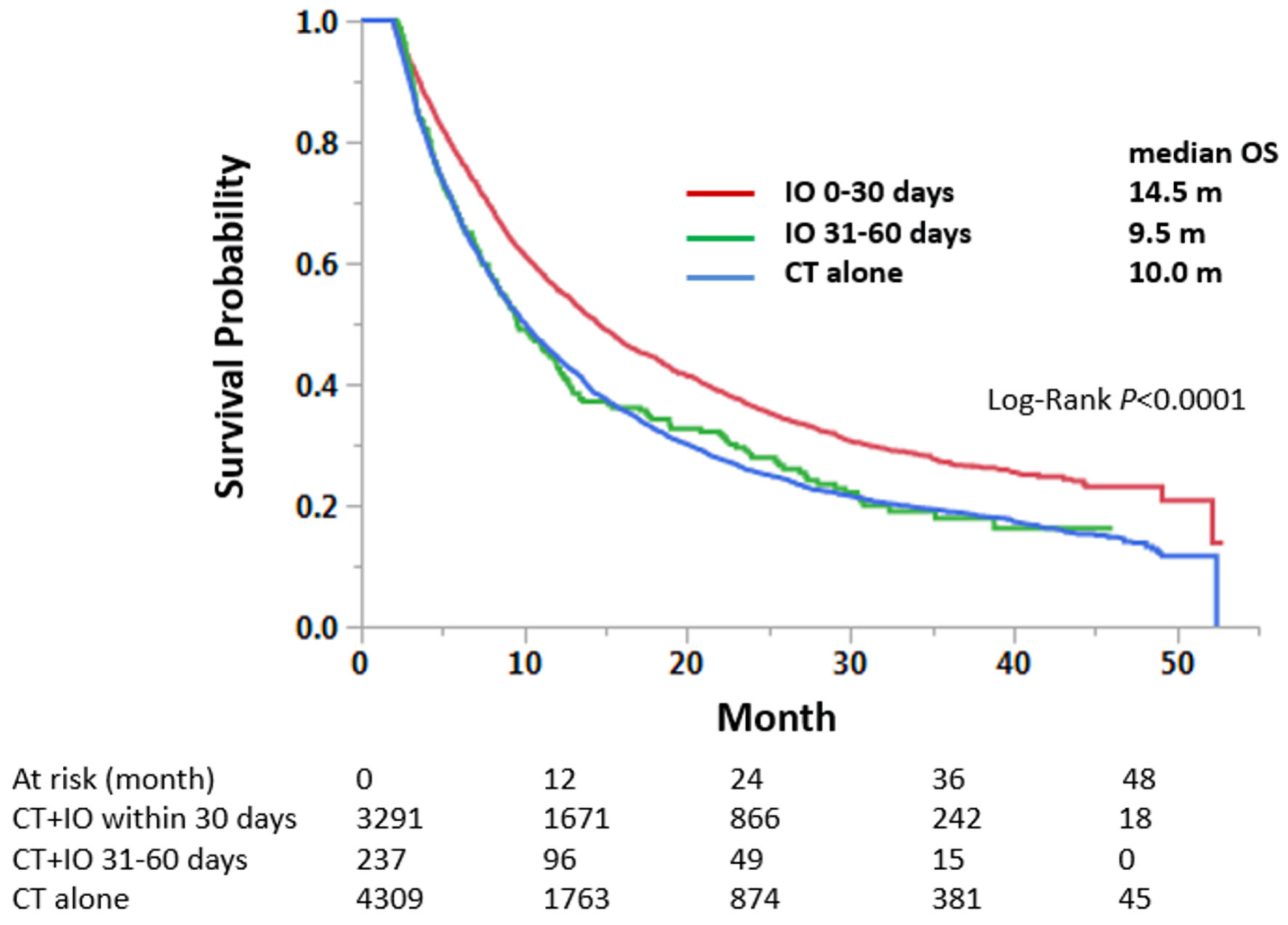
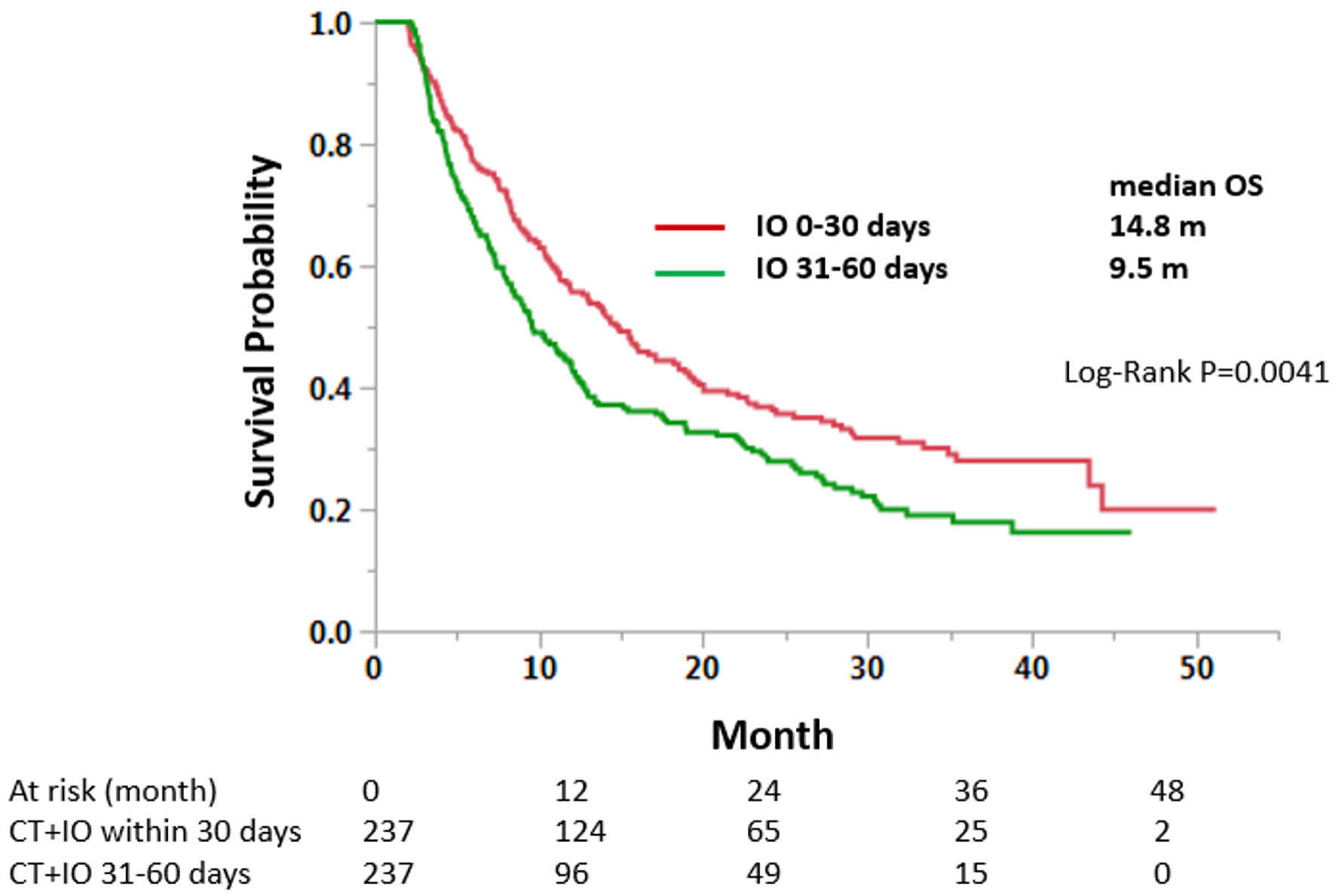
Tables
| Factors | Immunotherapy | Total | P value | ||
|---|---|---|---|---|---|
| Yes | Yes | No | |||
| Starting in | |||||
| 0 - 30 days | 31 - 60 days | NA | |||
| CD: Charlson-Deyo. | |||||
| Total | 3,291 (100%) | 237 (100%) | 4,309 (100%) | 7,837 (100%) | |
| Institution | |||||
| Academic | 965 (29%) | 67 (28%) | 1,227 (28%) | 2,259 (29%) | 0.71 |
| Other | 2,326 (71%) | 170 (72%) | 3,082 (72%) | 5,578 (71%) | |
| Age | |||||
| 70 and older | 1,075 (33%) | 83 (35%) | 1,573 (37%) | 2731 (35%) | < 0.01 |
| Less than 70 | 2,216 (67%) | 154 (65%) | 2,736 (63%) | 5,106 (65%) | |
| Sex | |||||
| Male | 1,838 (56%) | 123 (52%) | 2,536 (59%) | 4,497 (57%) | < 0.01 |
| Female | 1,453 (44%) | 114 (48%) | 1,773 (41%) | 3,340 (43%) | |
| Race | |||||
| White | 2,789 (85%) | 212 (89%) | 3,540 (82%) | 6,541 (83%) | < 0.01 |
| Other | 502 (15%) | 25 (11%) | 769 (18%) | 1,296 (17%) | |
| CD score | |||||
| 0 - 1 | 3,138 (95%) | 226 (95%) | 4,131 (96%) | 7,495 (96%) | 0.54 |
| 2 - 3 | 153 (5%) | 11 (5%) | 178 (4%) | 342 (4%) | |
| Year of diagnosis | |||||
| 2017 | 1,164 (35%) | 116 (49%) | 2,809 (65%) | 4,089 (52%) | < 0.01 |
| 2018 | 2,127 (65%) | 121 (51%) | 1,500 (35%) | 3,748 (48%) | |
| Histology | |||||
| Adenocarcinoma | 2,785 (85%) | 159 (67%) | 2,634 (61%) | 5,578 (71%) | < 0.01 |
| Other | 506 (15%) | 78 (33%) | 1,675 (39%) | 2,259 (29%) | |
| Clinical T stage | |||||
| cT3-4 | 1,554 (47%) | 129 (54%) | 2,214 (51%) | 3,897 (50%) | < 0.01 |
| Other | 1,737 (53%) | 108 (46%) | 2,095 (49%) | 3,940 (50%) | |
| Clinical N stage | |||||
| cN2-3 | 2,250 (68%) | 153 (65%) | 2,905 (67%) | 5,308 (68%) | 0.39 |
| Other | 1,041 (32%) | 84 (35%) | 1,404 (33%) | 2,529 (32%) | |
| Clinical M stage | |||||
| cM1BC | 2,240 (68%) | 162 (68%) | 2,790 (65%) | 5,192 (66%) | < 0.01 |
| Other | 1,051 (32%) | 75 (32%) | 1,519 (35%) | 2,645 (34%) | |
| Surgery | |||||
| Yes | 63 (2%) | 4 (2%) | 69 (2%) | 136 (2%) | 0.58 |
| No | 3,228 (98%) | 233 (98%) | 4,240 (98%) | 7,701 (98%) | |
| Radiation | |||||
| Yes | 1,237 (38%) | 116 (49%) | 1,947 (45%) | 3,300 (42%) | < 0.01 |
| No | 2,054 (62%) | 121 (51%) | 2,362 (55%) | 4,537 (58%) | |
| Brain metastasis | |||||
| Yes | 771 (23%) | 52 (22%) | 918 (21%) | 1,741 (22%) | 0.09 |
| No | 2,520 (77%) | 185 (78%) | 3,391 (79%) | 6,096 (78%) | |
| Liver metastasis | |||||
| Yes | 651 (20%) | 49 (21%) | 869 (20%) | 1,569 (20%) | 0.89 |
| No | 2,640 (80%) | 188 (79%) | 3,440 (80%) | 6,268 (80%) | |
| Factors | Univariate | Multivariate |
|---|---|---|
| HR (95% CI), P value | HR (95% CI), P value | |
| NSCLC: non-small cell lung cancer; HR: hazard ratio; CI: confidence interval; CD: Charlson comorbidity. | ||
| Institution | ||
| Academic | 0.92 (0.84 - 1.01), 0.07 | 0.94 (0.85 - 1.02), 0.14 |
| Others (reference) | ||
| Age | ||
| < 70 | 0.79 (0.72 - 0.86), < 0.01 | 0.78 (0.71 - 0.85), < 0.01 |
| ≥ 70 (reference) | ||
| Sex | ||
| Female | 0.79 (0.72 - 0.85), < 0.01 | 0.79 (0.73 - 0.86), < 0.01 |
| Male (reference) | ||
| Race | ||
| Others | 0.83 (0.73 - 0.93), < 0.01 | 0.86 (0.76 - 0.97), 0.01 |
| White (reference) | ||
| CD score | ||
| 0 - 1 | 1.01 (0.84 - 1.23), 0.92 | 0.95 (0.79 - 1.15), 0.63 |
| ≥ 2 (reference) | ||
| Year of diagnosis | ||
| 2018 | 0.99 (0.91 - 1.08), 0.82 | 1.00 (0.92 - 1.09), 0.99 |
| 2017 (reference) | ||
| Histology | ||
| Adenocarcinoma | 0.81 (0.73 - 0.90), < 0.01 | 0.88 (0.79 - 0.99), 0.02 |
| Others (reference) | ||
| Clinical T stage | ||
| Others | 0.92 (0.85 - 1.00), 0.05 | 0.96 (0.88 - 1.04), 0.34 |
| T3-4 (reference) | ||
| Clinical N Stage | ||
| Others | 0.83 (0.76 - 0.91), < 0.01 | 0.83 (0.76 - 0.91), < 0.01 |
| N2-3 (reference) | ||
| Clinical M stage | ||
| Other | 0.82 (0.75 - 0.90), <0.01 | 0.95 (0.86 - 1.04), 0.26 |
| M1BC (reference) | ||
| Surgery | ||
| Yes | 0.70 (0.50 - 0.95), 0.02 | 0.74 (0.53 - 1.03), 0.07 |
| No (reference) | ||
| Radiation | ||
| No | 0.84 (0.76 - 0.91), < 0.01 | 0.82 (0.75 - 0.91), < 0.01 |
| Yes (reference) | ||
| Brain metastasis | ||
| No | 0.93 (0.85 - 1.02), 0.13 | 0.99 (0.88 - 1.10), 0.81 |
| Yes (reference) | ||
| Liver metastasis | ||
| No | 0.63 (0.58 - 0.70), < 0.01 | 0.64 (0.58 - 0.71), < 0.01 |
| Yes (reference) | ||
| Time of immunotherapy | ||
| 0 - 30 days | 0.74 (0.64 - 0.87), < 0.01 | 0.78 (0.67 - 0.91), < 0.01 |
| 31 - 60 days (reference) | ||