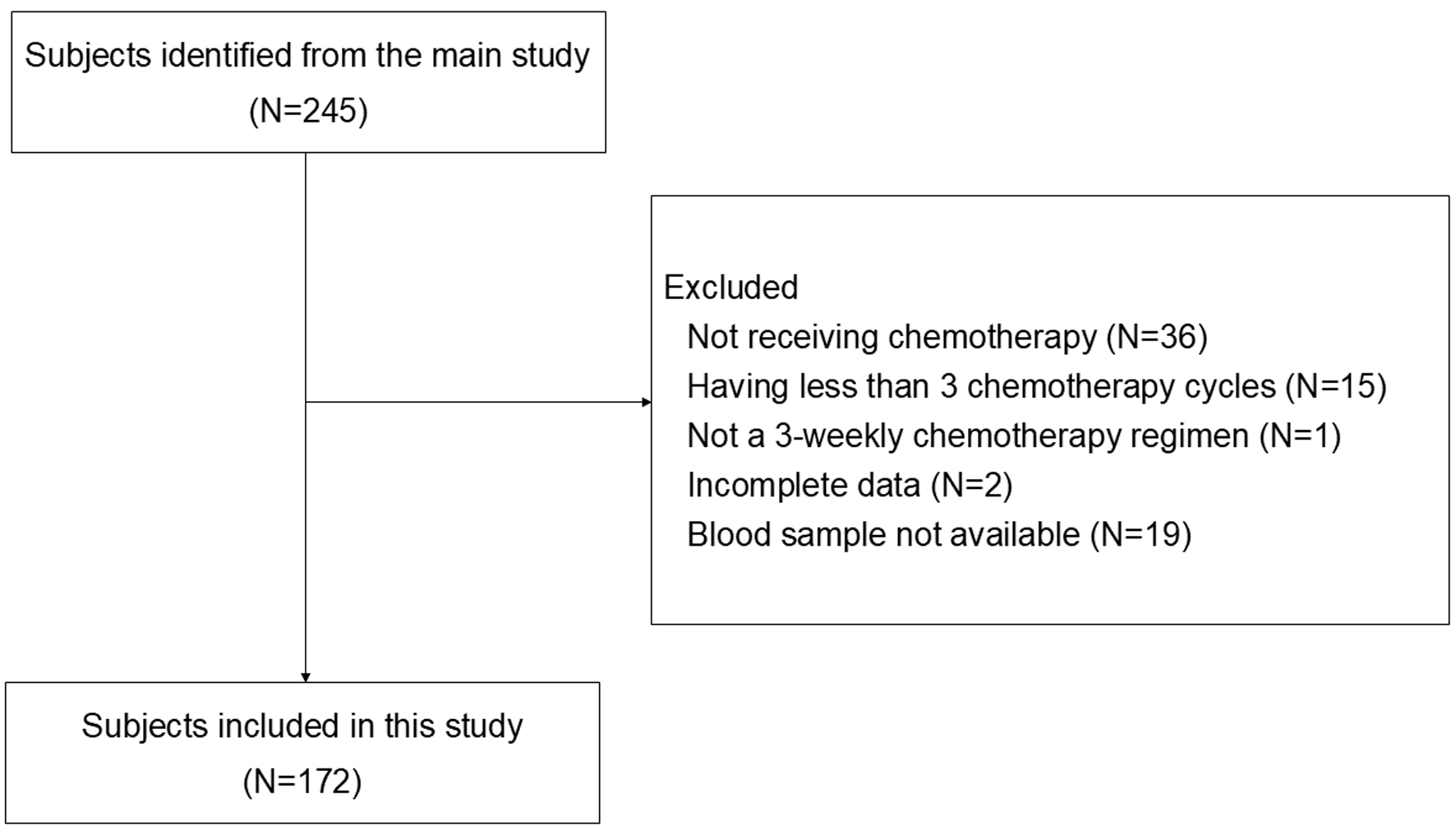
Figure 1. Flowchart of subjects’ inclusion. From 245 patients in the main study, 73 patients were excluded due to not receiving chemotherapy, receiving less than 3 chemotherapy cycles, not having 3-weekly chemotherapy regimen, incomplete clinical data, and unavailability of stored blood sample. A total of 172 patients with stage I-IV BC were included for analysis.