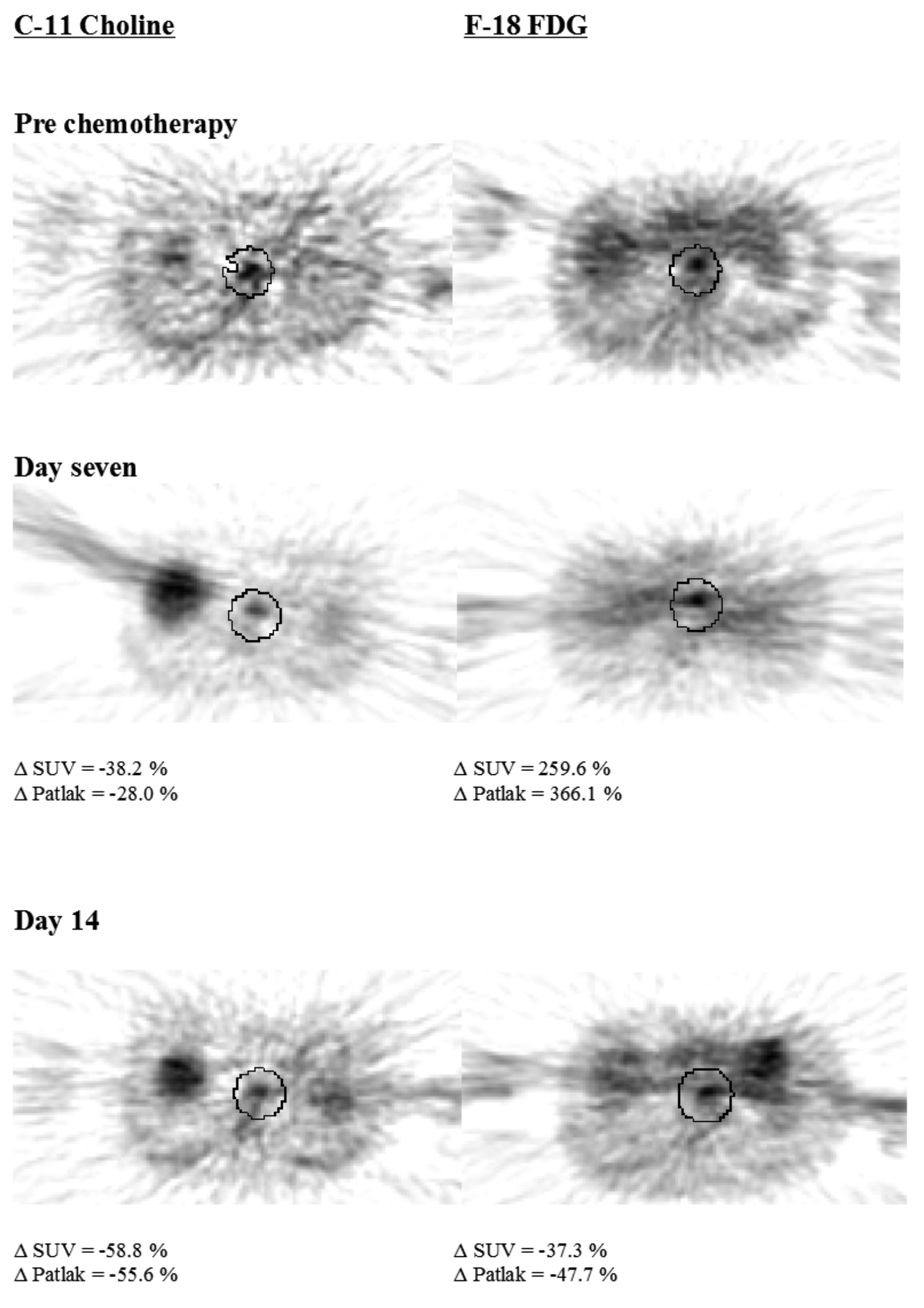
Figure 1. Trans-axial PET images of patient three, a pathological responder.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 1, Number 2, April 2010, pages 66-67
F-18-FDG and C-11-Choline Positron Emission Tomography in Human Esophago-Gastric Cancer: Prediction of Response to Therapy
Figures

Tables
| Patient | Sex | Age | Tumour Location | Tumour Histology | Pre Treatment AJCC Stage | Staging Modality | Chemotherapy | Chemotherapy Regime | Chemotherapy Cycles Completed | Re staging (CT) | Further Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (M = male; F = female; E = esophagus; EGJ = esophago-gastric junction; A = adenocarcinoma; S = squamous cell carcinoma; CT = computerised tomography; EUS = endoscopic ultrasound; E = epirubicin; C = cisplatin; F =5-fluorouracil; X = capecitabine; M = mitomycin-C; CRT = chemoradiotherapy) | |||||||||||
| 1 | M | 55.5 | E | A | III | CT | MCX | Palliative | 3 | - | - |
| 2 | M | 54.4 | EGJ | A | IIA | CT | CF | Neoadjuvant | 2 | IIA | Surgery |
| 3 | F | 67.6 | E | A | III | CT | CF | Neoadjuvant | 2 | - | Surgery |
| 4 | M | 50.9 | EGJ | A | IIA | CT | CF | Neoadjuvant | 2 | IIA | Surgery |
| 5 | M | 50.1 | E | S | IIA | CT/EUS | CF | Neoadjuvant | 2 | - | Surgery |
| 6 | F | 69.4 | E | A | IIA | CT | CX | Neoadjuvant | 2 | IIA | CRT |
| 7 | M | 56.5 | EGJ | A | III | CT/EUS | CX | Neoadjuvant | 2 | III | CRT |
| 8 | M | 61.9 | E | A | IVB | CT | ECF | Palliative | 0 | - | - |
| 9 | M | 78.1 | EGJ | A | IVB | CT | ECF | Palliative | 4 | IVB | ECF |
| 10 | F | 70.7 | E | A | III | CT | CX | Neoadjuvant | 2 | - | Surgery |
| 11 | M | 52.5 | EGJ | A | IVA | CT | CX | Neoadjuvant | 2 | - | Surgery |
| 12 | M | 54.3 | E | A | IIA | CT/EUS | CX | Neoadjuvant | 2 | IIA | Surgery |
| 13 | M | 63.3 | EGJ | A | IIA | CT | ECX | Neoadjuvant | 3 | III | CRT |
| 14 | F | 60.0 | EGJ | A | IVB | CT | MCX | Palliative | 3 | IVB | MCX |
| 15 | F | 67.9 | E | S | IVA | CT | MCX | Neoadjuvant | 3 | III | Surgery |
| 16 | F | 64.7 | E | A | III | CT | CX | Neoadjuvant | 2 | III | Surgery |
| 17 | M | 57.9 | E | A | III | CT | CX | Neoadjuvant | 2 | IVA | CRT |
| 18 | M | 59.7 | EGJ | A | III | CT | ECX | Neoadjuvant | 3 | IVA | CRT |
| Patient | Chemotherapy Regime | TRG | Resection Status | Pathological Stage | Survival (months) | Status at Follow Up | Median Ki-67 Tumour Nucleii in pre-treatment biopsy (± SD) | High Power Fields Counted | Mean ki-67 Tumour Nuclei in Resection Specimen (± SD) | High Power Fields Counted | % Δ in Ki-67 Staining |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Palliative | 6.0 | Dead | 236 (55) | 5 | ||||||
| 2 | Neoadjuvant | 4 | R1 | T3 N1 Mx | 7.6 | Dead | |||||
| 3 | Neoadjuvant | 3 | R1 | T2 N1 Mx | 8.4 | Dead | 117 (48) | 5 | 85 (44) | 6 | -27.4 |
| 4 | Neoadjuvant | 3 | R0 | T3 N1 Mx | 15.8 | Dead | 228 (72) | 4 | 203 (41) | 5 | -11.0 |
| 5 | Neoadjuvant | 5 | R0 | T3 N0 Mx | 25.1 | Alive | 276 (39) | 5 | 184 (46) | 6 | -33.3 |
| 6 | Neoadjuvant | 24.4 | Alive | 395 (43) | 5 | ||||||
| 7 | Neoadjuvant | 17.9 | Dead | 186 (43) | 5 | ||||||
| 8 | Palliative | 0.3 | Dead | 741 (79) | 3 | ||||||
| 9 | Palliative | 9.2 | Dead | 466 (51) | 5 | ||||||
| 10 | Neoadjuvant | 4 | R1 | T3 N1 Mx | 11.9 | Dead | 575 (63) | 4 | 135 (24) | 15 | -76.5 |
| 11 | Neoadjuvant | 2 | R0 | T3 N1 Mx | 20.0 | Alive | 616 (31) | 4 | 73 (35) | 7 | -88.1 |
| 12 | Neoadjuvant | 4 | R1 | T3 N1 Mx | 19.8 | Alive | 194 (82) | 5 | 149 (30) | 7 | -23.2 |
| 13 | Neoadjuvant | 18.2 | Alive | 437 (107) | 5 | ||||||
| 14 | Palliative | 10.0 | Dead | 35 (5) | 2 | ||||||
| 15 | Neoadjuvant | 2 | R0 | T2 N0 Mx | 16.4 | Alive | 209 (42) | 5 | 0 (0) | 5 | -100.0 |
| 16 | Neoadjuvant | 4 | R0 | T3 N1 Mx | 15.4 | Alive | |||||
| 17 | Neoadjuvant | 13.8 | Alive | 536 (76) | 4 | ||||||
| 18 | Neoadjuvant | 13.7 | Alive | 437 (158) | 5 |
| Quantification | Tracer | Median Uptake (± SD) | Survival, months (± SD) | p | n |
|---|---|---|---|---|---|
| SUV | F-18 FDG | 0.17 (0.06) | < median 14.8 (2.0) > median 17.2 (2.7 | 0.70 | 18 |
| C-11 Choline | 0.15 (0.01) | < median 19.3 (2.7) > median 11.5 (2.3) | 0.11 | 16 |
| PET Timing | Tracer | Median % Δ SUV (± SD) | Survival, months (± SD) | p | n |
|---|---|---|---|---|---|
| Day 7 | F-18 FDG | -3.3 (81.9) | < Median 15.0 (2.3) > Median 20.7 (2.7) | 0.43 | 13 |
| C-11 Choline | 0.0 (24.1) | < Median 21.3 (3.4) > Median 14.1 (2.1) | 0.24 | 11 | |
| Day 14 | F-18 FDG | -17.1 (28.3) | < Median 18.1 (1.2) > Median 17.2 (3.3) | 0.41 | 12 |
| C-11 Choline | -0.2 (36.9) | < Median 21.1 (3.4) > Median 13.2 (2.1) | 0.16 | 9 |