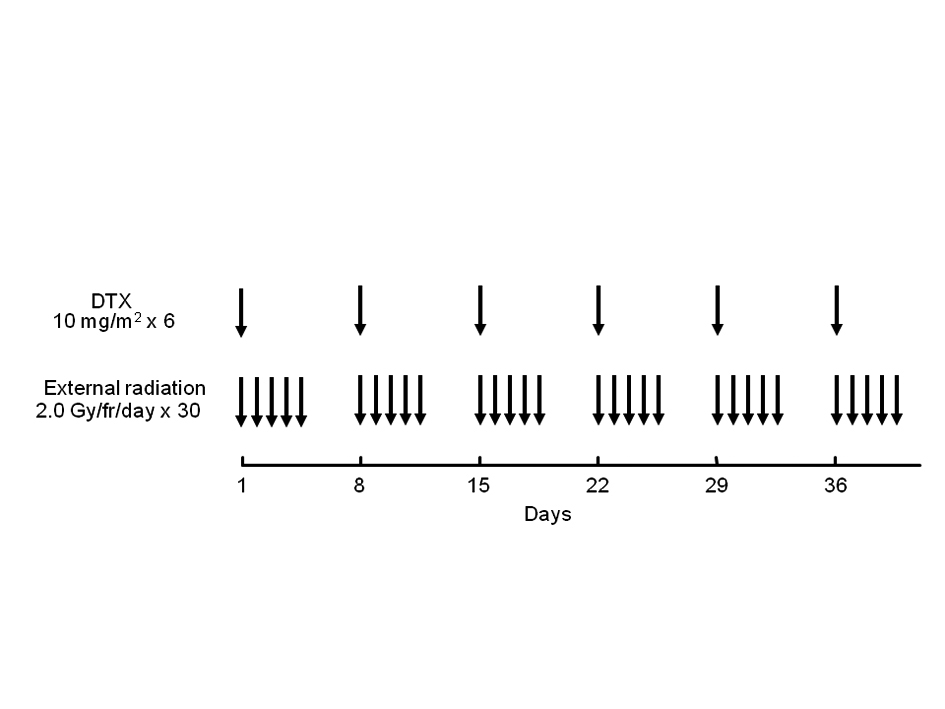
Figure 1. Treatment schedule of chemoradiotherapy with weekly docetaxel. DTX: docetaxel.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 2, Number 5, October 2011, pages 252-258
A Pilot Study of Chemoradiotherapy With Weekly Docetaxel for Thoracic Esophageal Carcinoma With T4 and/or M1 Lymph Node Metastasis
Figures

Tables
| Characteristic | n = 9 |
|---|---|
| Age (years) | |
| Median | 66 |
| Range | 51-78 |
| Gender | |
| Female | 1 |
| Male | 8 |
| Performance status | |
| 0 | 7 |
| 1 | 2 |
| UICC TNM stage | |
| non-T4 M1 LYM | 4 |
| T4 M0 | 4 |
| T4 M1 LYM | 1 |
| Primary tumor site | |
| Upper thoracic esophagus | 3 |
| Middle thoracic esophagus | 5 |
| Lower thoracic esophagus | 1 |
| Site of M1 LYM disease | |
| Cervical node | 4 |
| Cervical and abdominal node | 1 |
| Grade (n = 9) | % ≥ Grade 3 | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Acute toxicity | ||||||
| Hematological | ||||||
| Leukopenia | 8 | 0 | 1 | 0 | 0 | 0 |
| Neutropenia | 8 | 0 | 1 | 0 | 0 | 0 |
| Anemia | 4 | 3 | 1 | 0 | 0 | 0 |
| Thrombocytopenia | 8 | 0 | 1 | 0 | 0 | 0 |
| Biochemical | ||||||
| Bilirubin | 9 | 0 | 0 | 0 | 0 | 0 |
| AST | 7 | 2 | 0 | 0 | 0 | 0 |
| ALT | 7 | 2 | 0 | 0 | 0 | 0 |
| Creatinine | 8 | 1 | 0 | 0 | 0 | 0 |
| Non-hematological | ||||||
| Anorexia | 6 | 1 | 0 | 2 | 0 | 22 |
| Nausea | 9 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 8 | 0 | 1 | 0 | 0 | 0 |
| Esophagitis | 6 | 1 | 0 | 2 | 0 | 22 |
| Esophageal bleeding | 7 | 0 | 1 | 1 | 0 | 11 |
| Dermatitis | 7 | 1 | 1 | 0 | 0 | 0 |
| Late toxicity | ||||||
| Peumonitis | 9 | 0 | 0 | 0 | 0 | 0 |
| Pleural effusion | 8 | 0 | 0 | 1 | 0 | 11 |
| Pericardial effusion | 9 | 0 | 0 | 0 | 0 | 0 |
| Case No. | Age | Gender | UICC TNM | Tumor site | Tumor size (cm) | Overall response | Primary response | Prognosis |
|---|---|---|---|---|---|---|---|---|
| PD, progressive disease; CR, complete response; PR, partial response; IR, incomplete response; SD, stable disease. | ||||||||
| 1 | 54 | M | T4 M0 | Middle | 8 | PD | PD | 8 M died of disease |
| 2 | 78 | M | T4 M0 | Upper | 12 | CR | CR | 27 M died of esophageal perforation |
| 3 | 60 | M | T4 M0 | Middle | 15 | PD | PD | 2 M died of disease |
| 4 | 51 | M | T1 M1 LYM | Lower | 5 | PD | CR | 36 M alive with disease progression |
| 5 | 66 | M | T1 M1 LYM | Middle | 3 | PR | CR | 29 M died of disease |
| 6 | 66 | F | T3 M1 LYM | Middle | 12 | CR | CR | 16 M died of disease |
| 7 | 70 | M | T3 M1 LYM | Middle | 10 | PR | IR/SD | 5 M alive with disease progression |
| 8 | 58 | M | T4 M1 LYM | Upper | 6 | PR | IR/SD | 9 M died of disease |
| 9 | 70 | M | T4 M0 | Upper | 13 | PR | IR/SD | 4 M died of disease |
| Number of patients | 9 |
|---|---|
| CR, complete response; PR, partial response; CI, confidence interval. | |
| Response rate | 67% (CR 22%; PR 44%) |
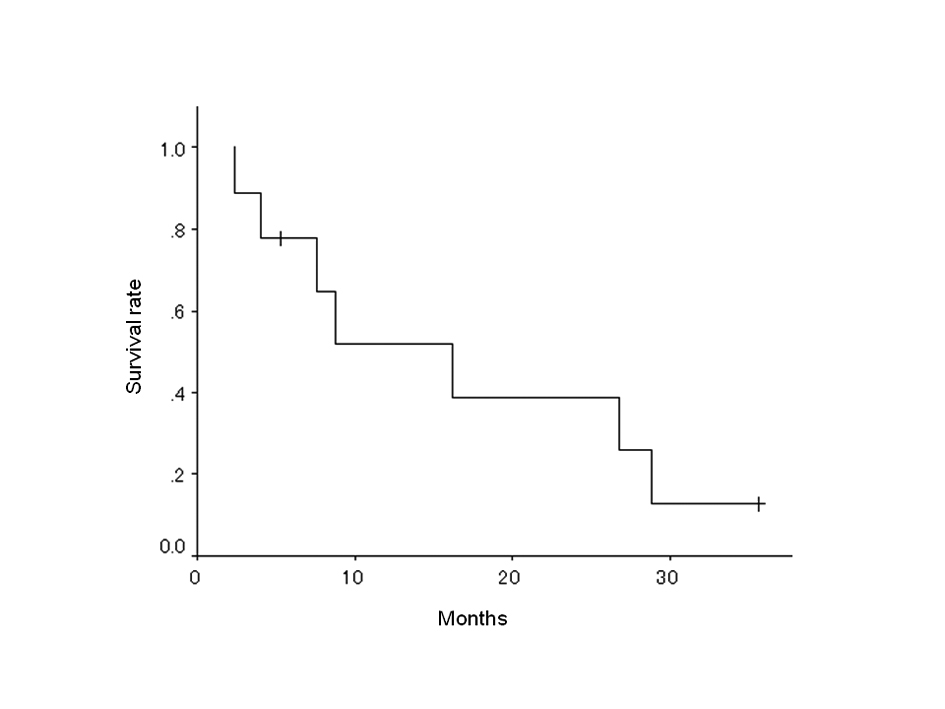
| Median survival time | 16.2 months (95% CI: 4.9-27.5) |
| 2-year survival rate | 38.9% |