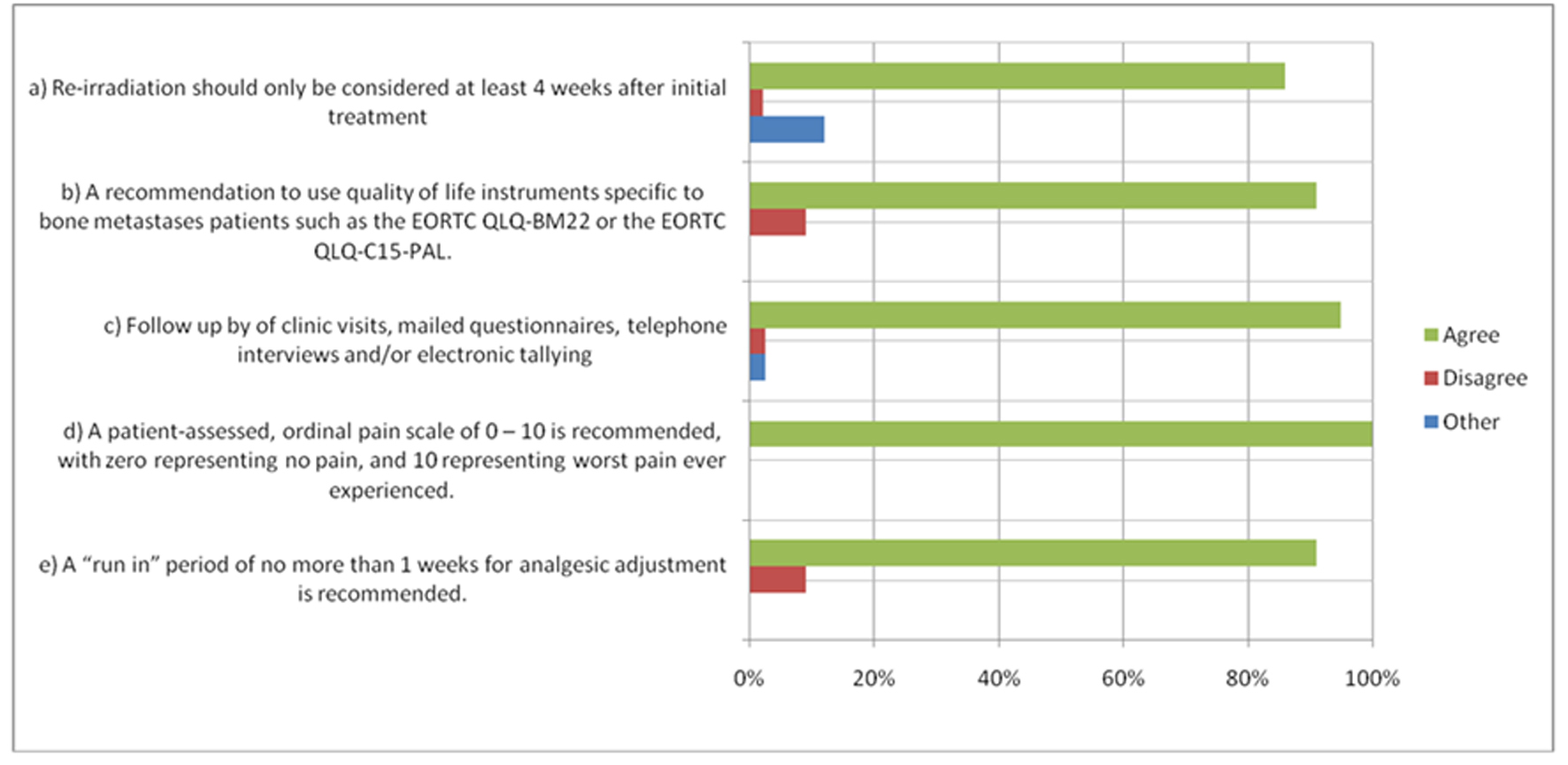
Figure 1. Items with > 80% Agreement. *Items with > 80% agreement as per the 2011 International Consensus. For other items, please see publication (1).
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Review
Volume 3, Number 1, February 2012, pages 1-7
New Considerations in the Design of Clinical Trials for Bone Metastases
Figure

Table
| Term | Definition |
|---|---|
| Complete Response | A pain score of zero at treated site with no concomitant increase in analgesic intake (stable or reducing analgesics in daily oral morphine equivalent (OMED)) |
| Partial Response | Pain reduction of 2 or more at the treated site on a 0 - 10 scale without analgesic increase, or Analgesic reduction of 25% or more from baseline without an increase in pain. |
| Pain Progression | Increase in pain score of 2 or more above baseline at the treated site with stable OMED, or An increase of 25% or more in OMED compared with baseline with the pain score stable or 1 point above baseline |
| Indeterminate Response | Any response that is not captured by the complete response, partial response or pain progression definitions. |