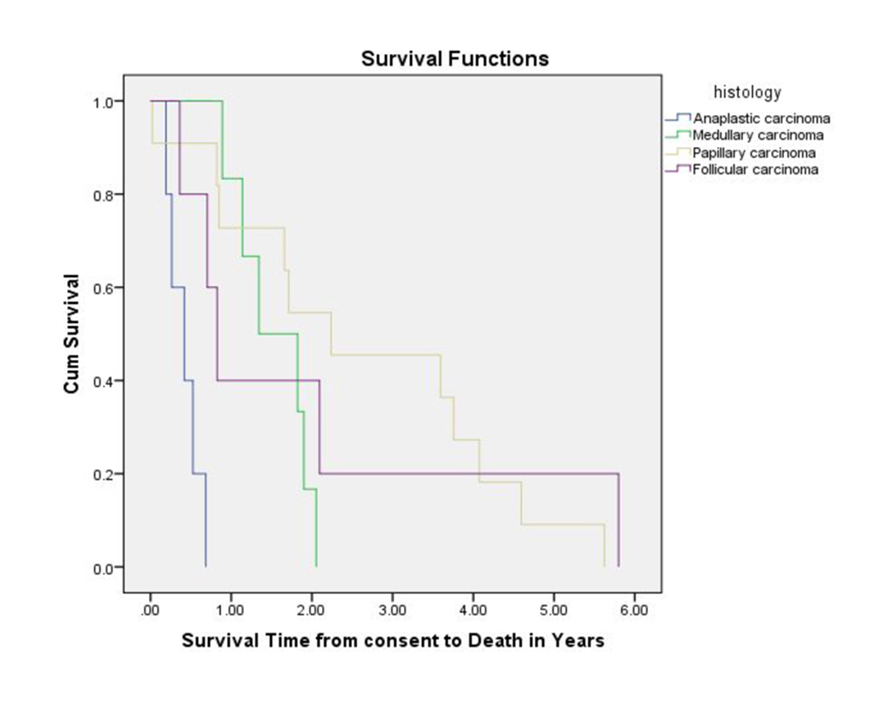
Figure 1. Overall survival measured from Phase I study enrollment until death.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 5, Number 1, February 2014, pages 24-32
Advanced Thyroid Cancer Patients in Phase I Clinical Trials: Outcome Assessment and Literature Review
Figure

Tables
| Age | |
|---|---|
| Median (range) | 56 (21-81) |
| Sex (no, %) | |
| Female | 15 (46.8) |
| Male | 17 (53.1) |
| Ethnicity (no, %) | |
| White | 11 (34.3) |
| Hispanic | 14 (43.7) |
| Other | 7 (21.8) |
| ECOG performance status (no, %) | |
| 0 | 15 (46.8) |
| ≥1 | 17 (53.1) |
| Tumor histology (no, %) | |
| Papillary | 12 (37.5) |
| Follicular | 6 (18.7) |
| Medullary | 9 (28.1) |
| Anaplastic | 5 (15.6) |
| No of metastatic sites (no, %) | |
| 1 | 12 (37.5) |
| 2 | 10 (31.3) |
| ≥3 | 10 (31.3) |
| Site of metastases (no, %) | |
| Lung | 22 (68.7) |
| Bone | 12 (37.5) |
| Lymph node | 10 (31.2) |
| Liver | 7 (21.8) |
| Mediastinum | 7 (21.8) |
| No of prior systemic treatments (no, %) | |
| 0 | 6 (18.7) |
| 1 | 18 (56.2) |
| ≥2 | 8 (25) |
| Type of prior treatment (no, %) | |
| Thyroidectomy | 32 (100) |
| Iodine-131 | 21 (65.6) |
| Chemotherapy | 19 (59.3) |
| Biological | 10 (31.2) |
| Type of Phase I trial (no, %) | |
| Single biological agent | 24 (75.0) |
| Combination biological therapy | 6 (18.7) |
| Combination of Chemotherapy and biological agent | 2 (6.2) |
| Reason to come off study (no, %) | |
| Progression | 20 (62.5) |
| Toxicity | 5 (15.6) |
| Patient preference/Other | 7 (21.8) |
| Treatment on progression (no, %) | |
| Another trial | 13 (40.6) |
| Off trial treatment | 8 (25.0) |
| No treatment/supportive care | 7 ( 21.8) |
| Unknown | 4 (12.5) |
| Phase I Drug target | Tumor type | Best response | Duration of response in Phase I trial (weeks) | Duration of response on prior therapy (weeks) |
|---|---|---|---|---|
| Abbreviations: MTD: Maximally tolerated dose; PTC: Papillary thyroid cancer; MTC: Medullary thyroid cancer; ATC: Anaplastic thyroid cancer; FTC: Follicular thyroid cancer; PR: Partial response; SD: Stable disease; HDAC: Histone deacetylase; VEGFR: Vascular endothelial growth factor receptor; EGFR: Epidermal growth factor receptor; HER2: Human epidermal growth factor receptor 2; mTOR: Mammalian target of rapamycin; IGF-IR: Insulin-like growth factor receptor; NA: Not available) | ||||
| HDAC | PTC | PR | 196 | 6 |
| VEGFR | MTC | PR | 72 | 8 |
| EGFR | ATC | SD | 9 | NA |
| HDAC | PTC | SD | 24 | 20 |
| HDAC | MTC | SD | 35 | 22 |
| mTOR | MTC | SD | 33 | NA |
| EGFR/HER2 | MTC | SD | 25 | 14 |
| EGFR/HER2 | MTC | SD | 14 | 16 |
| EGFR/HER2 | MTC | SD | 30 | 18 |
| mTOR | FTC | SD | 20 | 15 |
| EGFR | ATC | SD | 17 | 4 |
| EGFR | PTC | SD | 16 | 9 |
| VEGFR | PTC | SD | 42 | NA |
| VEGFR | FTC | SD | 48 | 6 |
| Microtubule | MTC | SD | 12 | 10 |
| VEGF | PTC | SD | 9 | 20 |
| IGF-IR | FTC | SD | 29 | 22 |
| IGF-IR | MTC | SD | 36 | 16 |
| EGFR | MTC | SD | 24 | 14 |