Figures
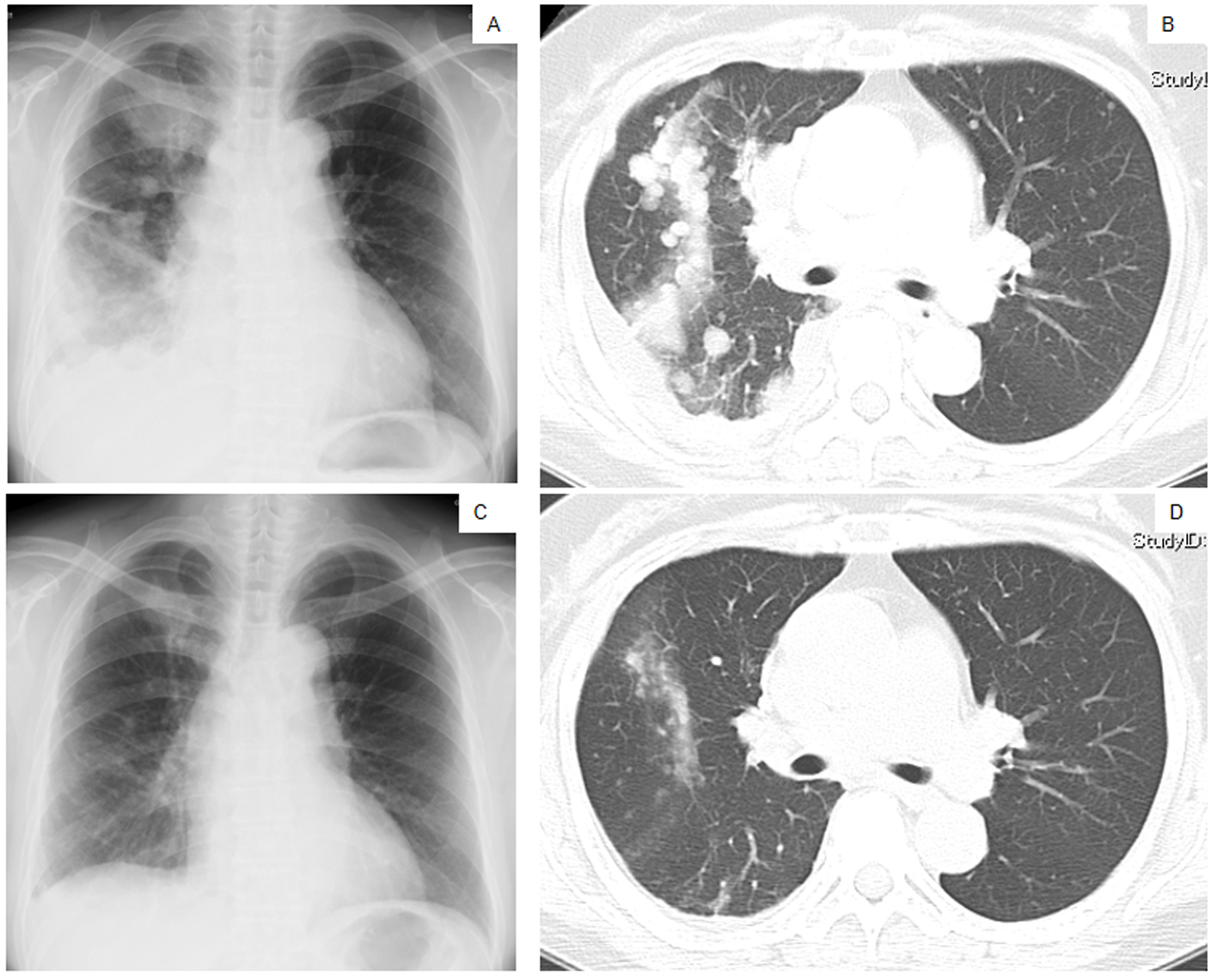
Figure 1. Chest X-ray films (A, C) and a CT scan of the middle lung fields (B, D) taken in April 2010 before first-line chemotherapy using carboplatin plus weekly paclitaxel (A, B), and taken in May 2010 after the second course of the first-line chemotherapy (C, D).
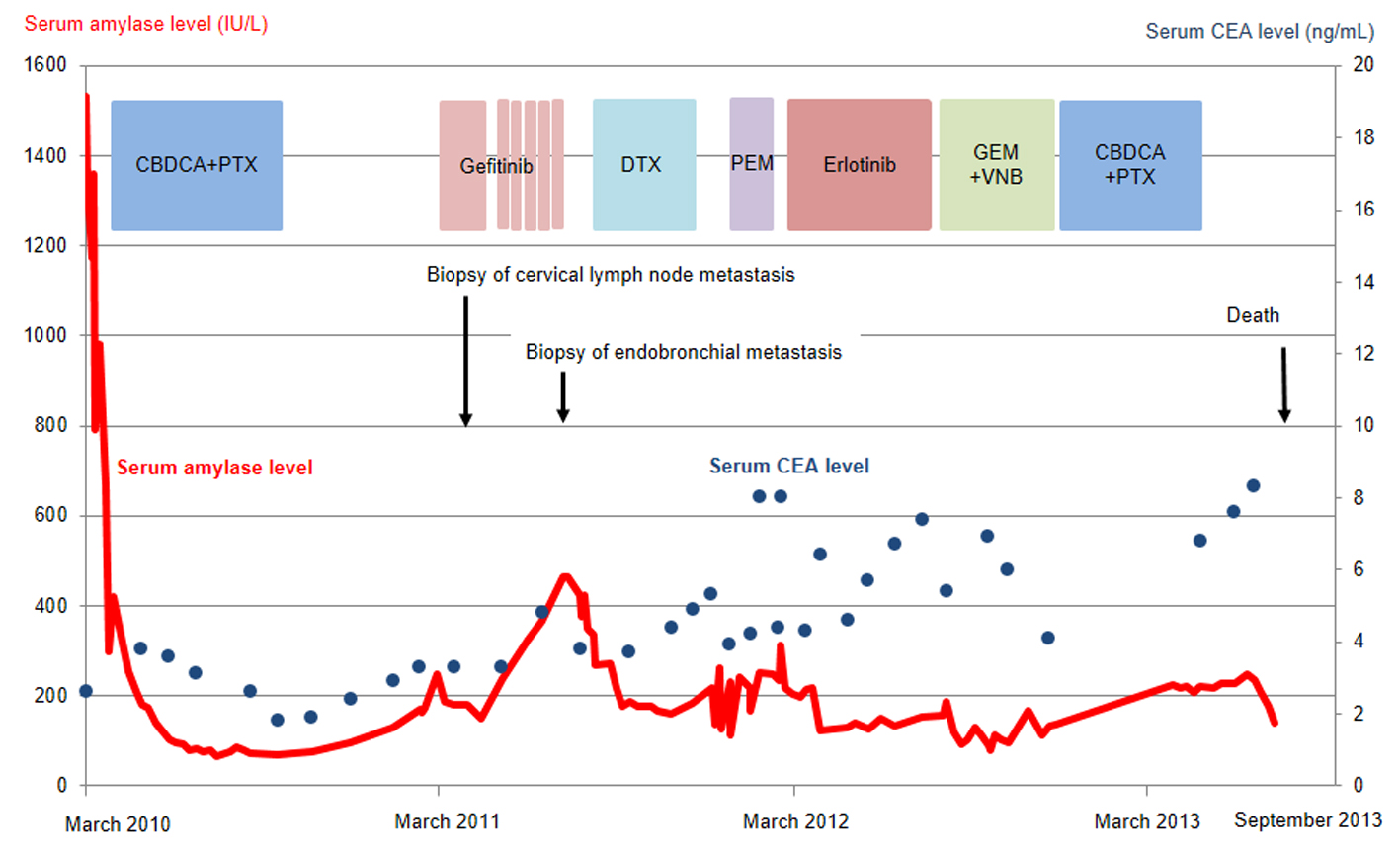
Figure 2. Serum amylase (red line) and CEA (blue dots) levels as a function of chemotherapy regimens. CBDCA, carboplatin; PTX, paclitaxel; DTX, docetaxel; PEM, pemetrexed; GEM, gemcitabine; VNB, vinorelbine; CEA, carcinogenic embryonic antigen.
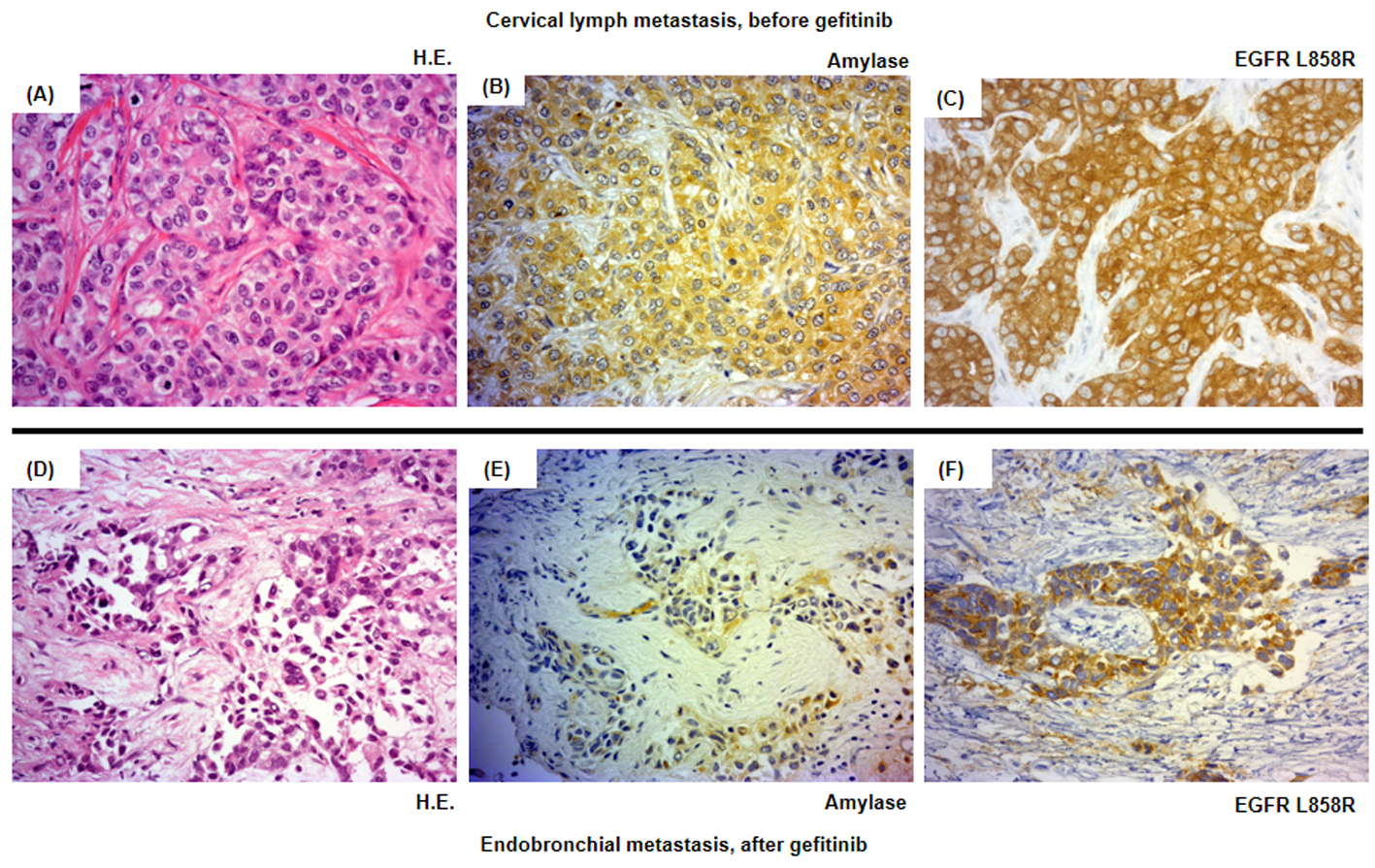
Figure 3. Histological analysis of the patient’s tumor cells. Magnification × 500. (A-C) Cervical lymph metastasis resected in March 2011 before the introduction of the second-line gefitinib treatment. (D-F) Endotracheal metastasis in August 2011 after progressive disease following gefitinib treatment. (A, D) Hematoxylin and eosin staining (HE staining). (B, E) Salivary-type amylase staining. (C, F) EGFR (L858R mutation-specific) staining.
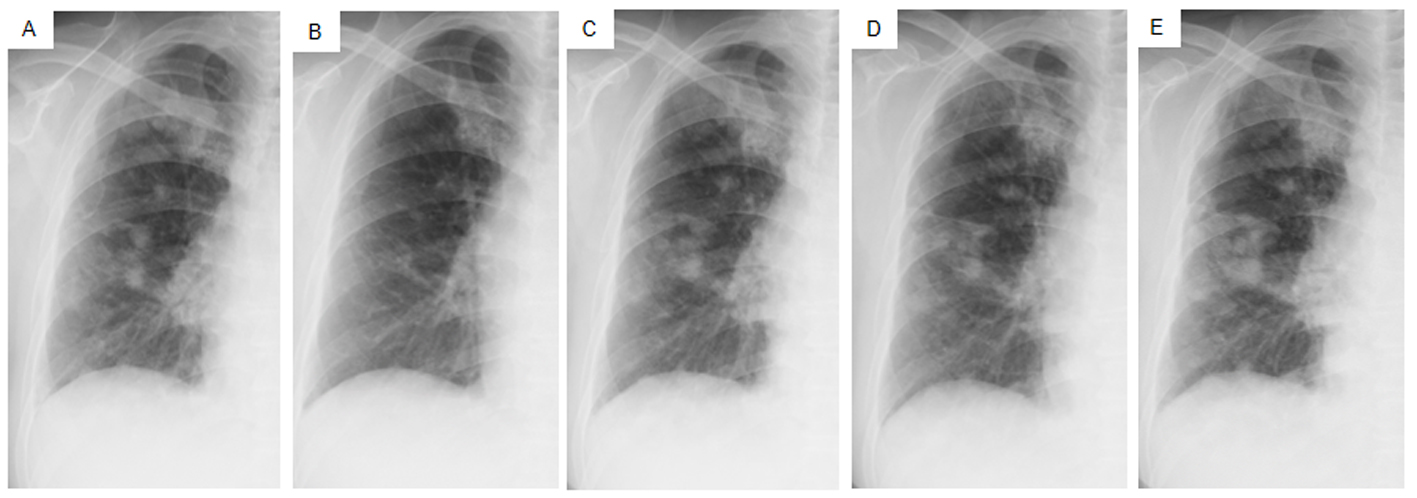
Figure 4. Chest X-ray films of the right lung field taken late in March 2011 before the administration of gefitinib (A), taken early in May at 1.5 months after initiating gefitinib and just before the suspension of gefitinib due to grade 3 elevation of alanine aminotransferase levels (B), taken early in June at the resumption of gefitinib on alternative days (C), taken in mid-July at the 1.5 months after the resumption of gefitinib (D), taken in mid-August after the documentation of progressive disease and 4 days after discontinuation of gefitinib (E).



