| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 8, Number 3, June 2017, pages 86-91
Intrahepatic Biliary Metastasis of Colonic Adenocarcinoma: A Case Report With Immunohistochemical Analysis
Keisuke Kawashimaa, Naoki Watanabeb, Sho Tawadac, Takahito Adachic, Makoto Yamadac, Yusuke Kitoha, Tamotsu Takeuchia, Takuji Tanakab, d
aDepartment of Pathology and Translational Research, Gifu University School of Mediine, 1-1 Yanagido, Gifu City, Gifu 501-1194, Japan
bDepartment of Diagnostic Pathology (DDP) & Research Center of Diagnostic Pathology (RC-DiP), Gifu Municipal Hospital, 7-1 Kashima-cho, Gifu City, Gifu 500-8513, Japan
cDepartment of Gastrointestinal Surgery, Gifu Municipal Hospital, 7-1 Kashima-cho, Gifu City, Gifu 500-8513, Japan
dCorresponding Author: Takuji Tanaka, Department of Diagnostic Pathology (DDP) & Research Center of Diagnostic Pathology (RC-DiP), Gifu Municipal Hospital, 7-1 Kashima-cho, Gifu City, Gifu 500-8513, Japan
Manuscript accepted for publication May 17, 2017
Short title: Intrahepatic Biliary Metastasis of Colon Cancer
doi: https://doi.org/10.14740/wjon1037w
| Abstract | ▴Top |
Although intrabiliary metastasis of carcinoma in the liver is unusual, intraductal and/or intraepithelial spread of cancer cells along intrahepatic bile ducts is now well recognized as hepatic metastasis. However, several clinical and laboratory findings, including images, lead us to differentially diagnose from primary intrahepatic cholangiocarcinoma. We report here on a case of colonic adenocarcinoma that metastasized to the liver with spread along with the intrahepatic bile duct of S5/6 area. The patient was a 51-year-old man and clinically diagnosed liver metastasis of sigmoid colon cancer (tub2, pMP, ly1, v0, n0), which was diagnosed and treated by sigmoidectomy 7 years ago. The right hepatic lobectomy was performed in March 2016 and histopathological examination revealed that moderately differentiated adenocarcinoma proliferated along the epithelium of intrahepatic bile ducts. Immunohistochemistry (IHC) showed that cancer cells in the intrahepatic bile ducts were positive for CK20, CDX2, CK17 and CK19, but negative for CK7, MUC-5AC, MUC-2 and CA19-9. The findings were almost the same as those of the sigmoid colon cancer removed in July 2009. We finally diagnosed the liver tumor as intrahepatic biliary metastasis of sigmoid colon cancer. Patients with liver metastasis of cancer are hard to be detected biliary invasion and spread on diagnostic image examination. Knowledge of distinctive morphological and IHC features can help to accurately diagnose this rare intrahepatic biliary metastasis of colonic cancer in routine pathological diagnostic procedures.
Keywords: Colorectal cancer; Liver metastasis; Intrabiliary growth; Immunohistochemistry
| Introduction | ▴Top |
Liver is one of the most common sites among the visceral tissues of the carcinomatous hematogenous metastasis [1]. Colorectal cancer (CRC) tends to metastasize to the regional lymph nodes within the colon and distantly to the liver. Such metastatic lesions are usually nodular masses [2]. In rare cases, intrabiliary growth of metastatic CRC in the liver was reported with a frequency of 0.00067% in the United States [3]. Since biliary invasion and/or metastasis of CRC in the liver mimics primary intrahepatic biliary neoplasm [4], the incidence of biliary metastasis of CRC in the liver is underestimated [5]. However, intrabiliary growth of metastatic liver cancer originating from CRC is a still rare manifestation of metastatic liver malignancy [6-16]. We present here a case of CRC with an unusual metastatic pattern mimicking primary intrahepatic cholangiocarcinoma. We could reach an accurate diagnosis of liver intrabiliary metastasis of CRC in a Japanese gentleman using immunohistochemical technique.
| Case Report | ▴Top |
Seven years ago, a 51-year-old Japanese man received sigmoidectomy for the treatment of sigmoid colon cancer (stage I). It was histopathologically moderately differentiated adenocarcinoma (tub2, Fig. 1). He was intensively followed up after the operation. He was well controlled for his diabetes mellitus in Department of General Medicine of our hospital. On November 16, 2015, slight elevation of serum enzymes, alkaline phosphatase (ALP, 352 IU/L; normal range, 104 - 338 IU/L) and γ-glutamyltransferase (γ-GT, 160 IU/L; normal range, 0 - 47 IU/L) of hepatobiliary system was noted. A tumor marker, CEA (5.2 ng/mL; normal range, 0 - 4.9 ng/mL), was slightly elevated. Follow-up CT (Fig. 2a) and MRI (Fig. 2b) examinations revealed dilatation of small intrahepatic bile duct in the S5/6 area, suggesting intrahepatic cholangiocarcinoma or metastatic tumorous lesion. Clinical diagnosis based on the image analysis was intrahepatic cholangiocarcinoma or liver metastasis of CRC.
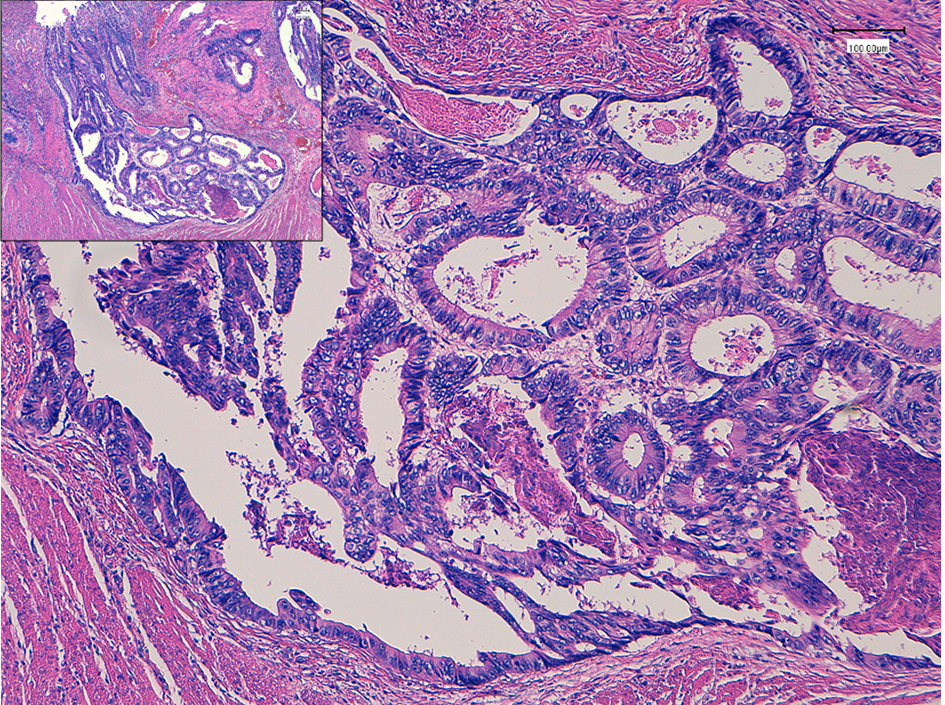 Click for large image | Figure 1. Histopathology of colon cancer, which was observed in the sigmoid colon 7 years ago. Moderately differentiated adenocarcinoma (tub2) invaded into the muscular layer. Insert is a low power of view. Bars, 100 μm. Hematoxylin and eosin stain. |
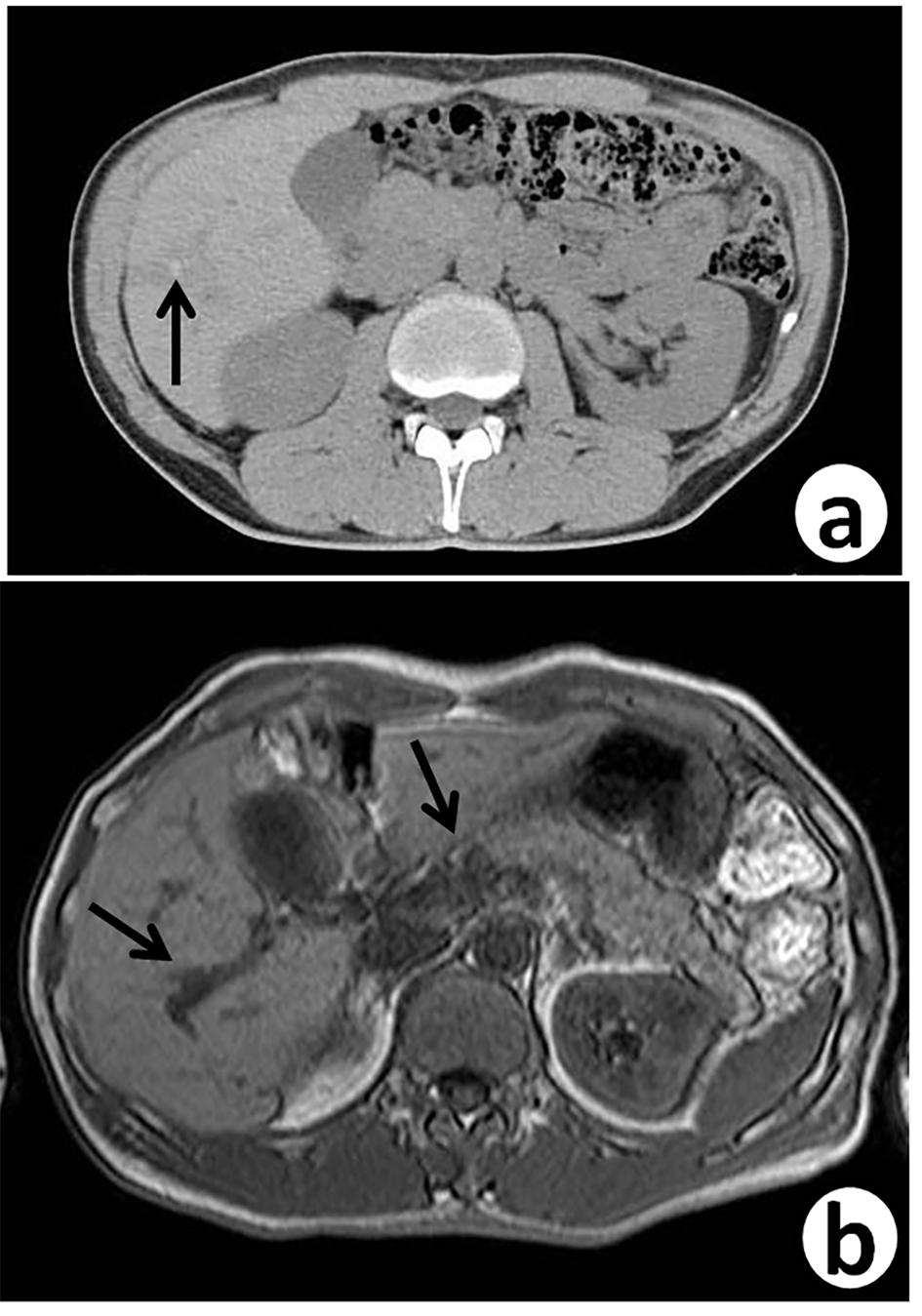 Click for large image | Figure 2. Follow-up abdominal contrast-enhanced CT scan (a) and MRI-T2 (b), 7 years after sigmoidectomy, reveal small dilated intrahepatic bile ducts (arrows) with thickening in the S5/6 area. |
He received right hepatic lobectomy on March 24, 2016. Macroscopically, small and whitish zonal lesions were noticed along with bile ducts (Fig. 3). Histopathological examination revealed that carcinoma cells showing moderately differentiated adenocarcinoma proliferated along the epithelium of intrahepatic bile ducts (Fig. 4a). Mass of cancer cells was present in the lumen of the intrahepatic bile ducts, and small foci of cancer cells were found in the normal bile duct epithelium (Fig. 4b). These findings suggested intrabiliary metastasis of adenocarcinoma, but not intrahepatic cholangiocarcinoma in the liver. Immunohistochemistry (IHC) using several antibodies, including CK7, CK17, CK19, CK20, CDX2, MUC-5AC, MUC-2, p53, CA19-9, and MIB-1 was performed to determine the origin of cancer cells. IHC findings were that cancer cells in the intrahepatic bile ducts were positive for CDX2 (Fig. 5a), p53 (Fig. 5b), CK17 (focal), and CK19 (focal), but negative for CK7 (Fig. 5c), CK20 (Fig. 5d), MUC-5AC, MUC-2 and CA19-9. MIB-1 positive rate of cancer cells in the liver was 30%. We also performed IHC in the sigmoid colon cancer removed in July 2009. Colon cancer cells were immunohistochemically positive for CDX2 (Fig. 6a), p53 (Fig. 6b), CK17, CK19, and CK20 (focal, Fig. 6d). However, they were negative for CK7 (Fig. 6c), MUC-5AC, MUC-2, and CA19-9. The positive rate of MIB-1 was 50%. Comparison of IHC findings on cancer cells found in the liver and colon in conjunction with intrahepatic bile duct cells is summarized in Table 1. A small cancer cell focus (Fig. 4b) was immunohistochemically positive for p53 (Fig. 7a) and MIB-1 (Fig. 7b), but negative for CA19-9 (Fig. 7c). In contrast, epithelial cells in surrounding bile duct were negative for p53 (Fig. 7a) and MIB-1 (Fig. 7b), but positive for CA19-9 (Fig. 7c). In addition, we observed microscopic tumor thrombi in the intrahepatic bile ducts (Fig. 8a). The tumor cells in the thrombi were positive for CDX2 (Fig. 8b). Interestingly, epithelial cells in some intrahepatic bile ducts were replaced by metastatic colon cancer cells (Fig. 8a, b).
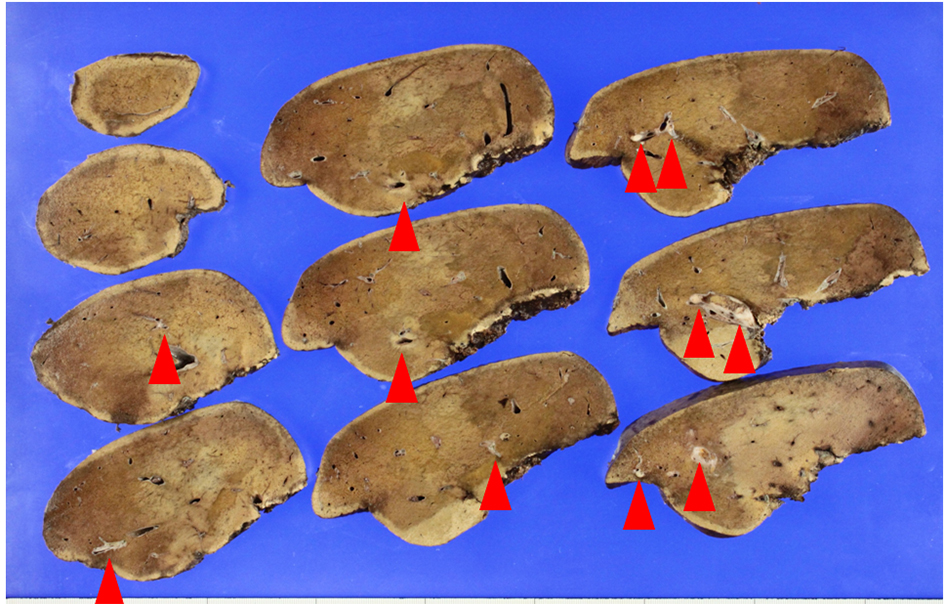 Click for large image | Figure 3. Macroscopic view of the lobectomized liver. Metastatic nodules (arrows) are found along with intrahepatic bile ducts. |
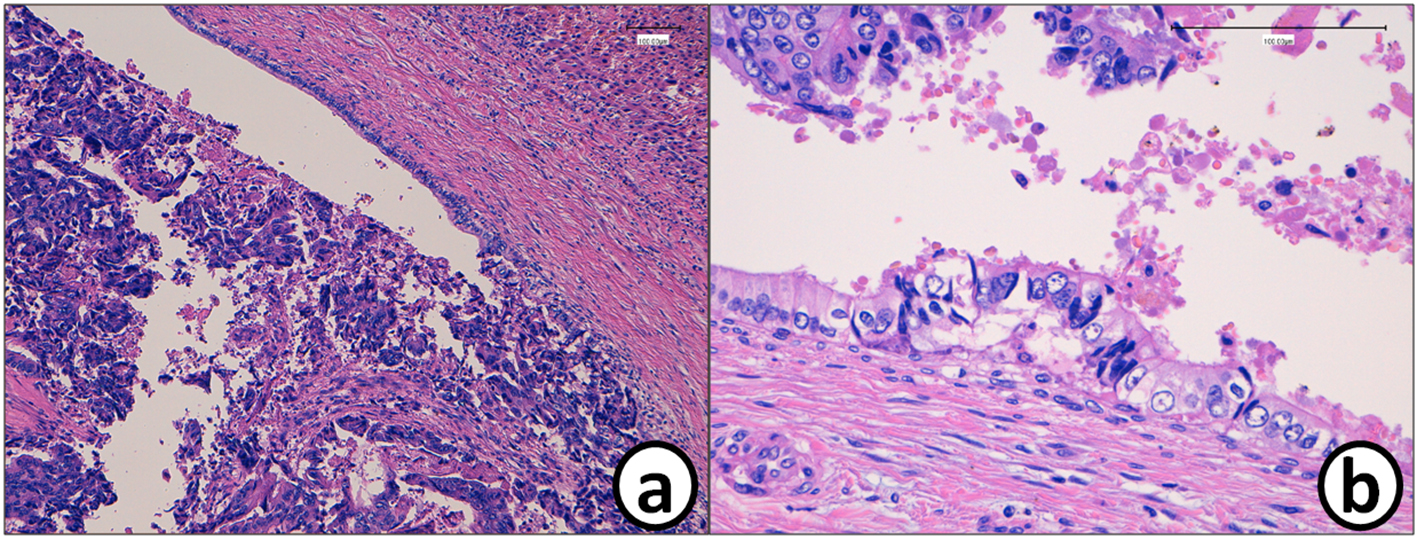 Click for large image | Figure 4. (a) Histopathology of the metastatic tumor in the liver (S5/6). (b) Mass of the cancer cells in the bile duct. Cancer cell mass is attached to the bile duct epithelium. A small metastatic focus is detected in the bile duct epithelium. Bars, 100 μm. Hematoxylin and eosin stain. |
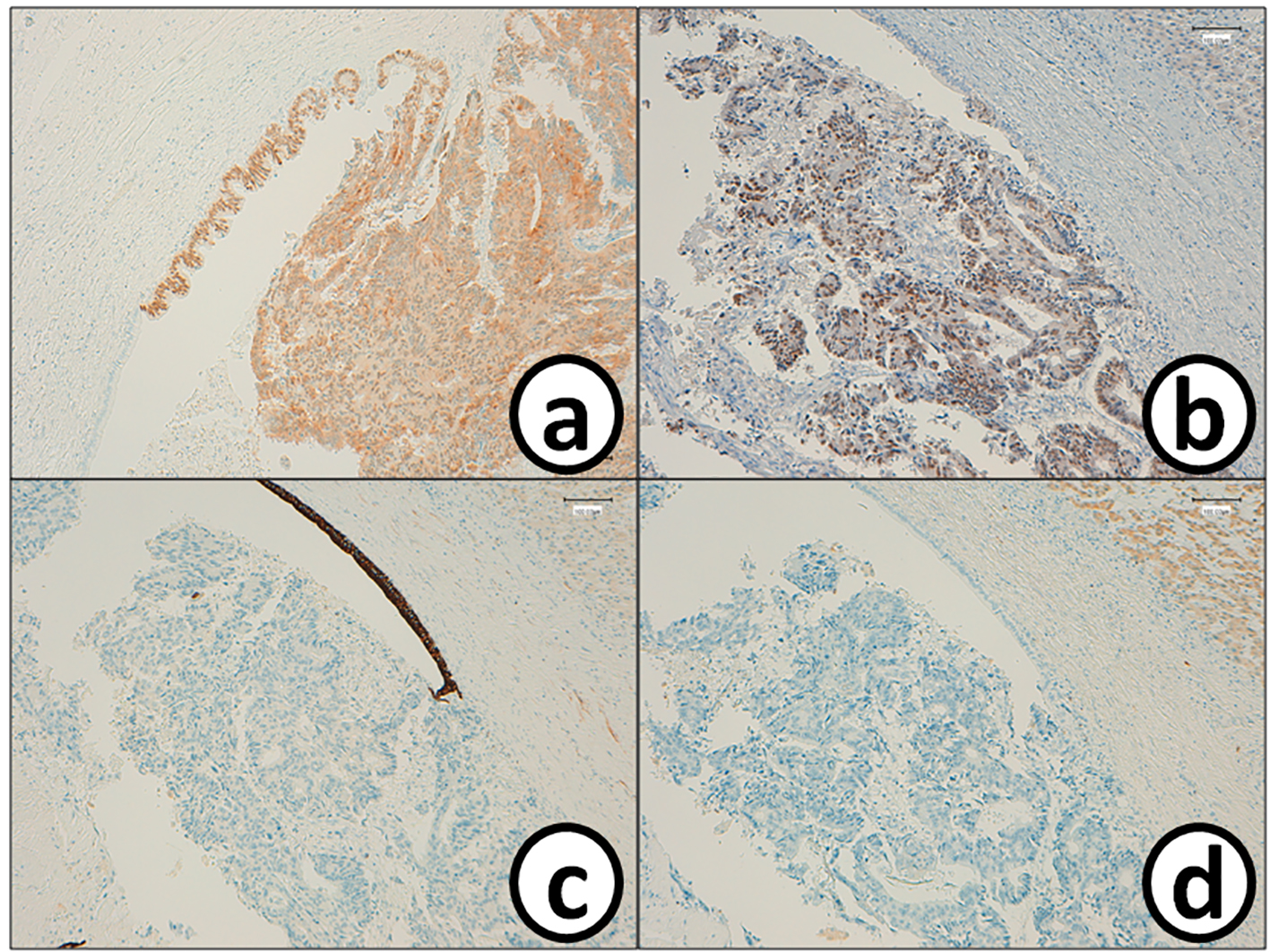 Click for large image | Figure 5. Metastatic cancer cells in the bile duct are positive for (a) CDX2 and (b) p53, while they are negative for (c) CK7 and (d) CK20. Bars, 100 μm. Immunohistochemistry for (a) CDX2, (b) p53, (c) CK7 and (d) CK20. |
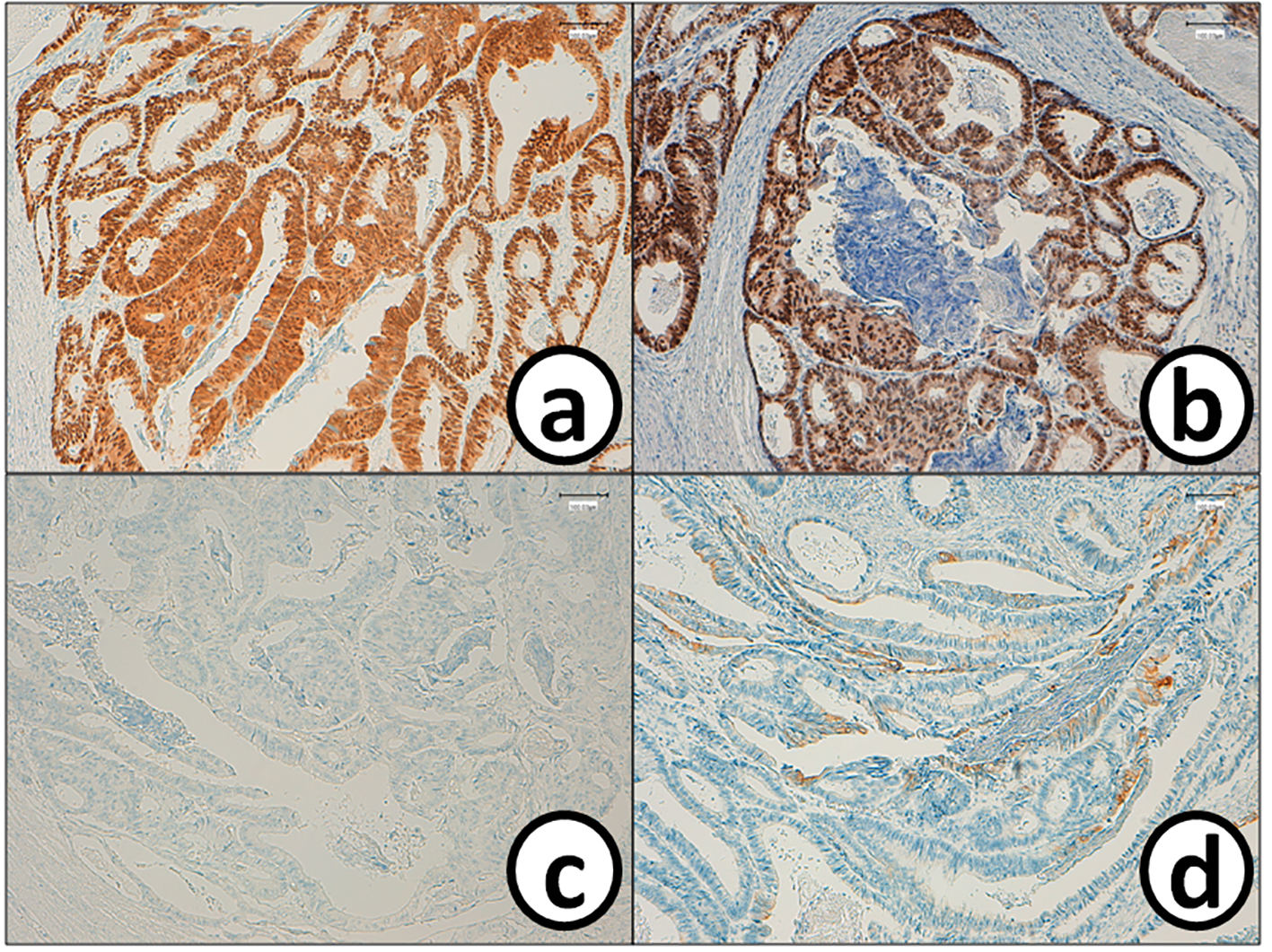 Click for large image | Figure 6. Cancer cells in the sigmoid colorectum are positive for (a) CDX2 and (b) p53, while they are negative for (c) CK7 and (d) CK20. Bars, 100 μm. Immunohistochemistry for (a) CDX2, (b) p53, (c) CK7 and (d) CK20. Bars, 100 μm. |
 Click to view | Table 1. Comparison of Immunohistochemical Findings |
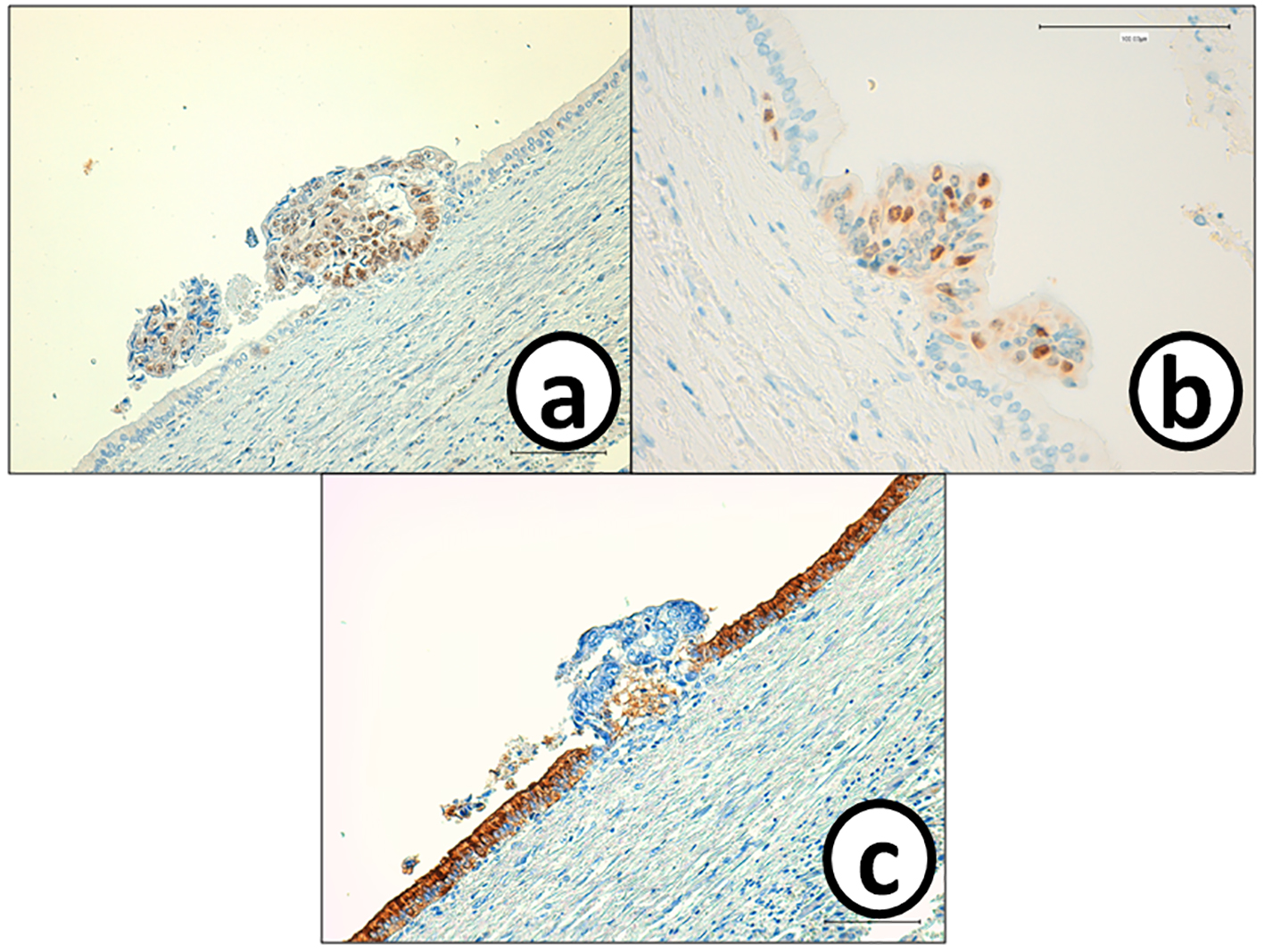 Click for large image | Figure 7. Cancer cells in the small metastatic focus in the bile duct epithelium are p53 and MIB-1, but negative for CA19-9. On the other hand, bile ducts epithelium is negative for (a) p53 and (b) MIB-1, but positive for (c) CA19-9. Immunohistochemistry of (a) p53, (b) MIB-1 and (c) CA-19-9. Bars, 100 μm. |
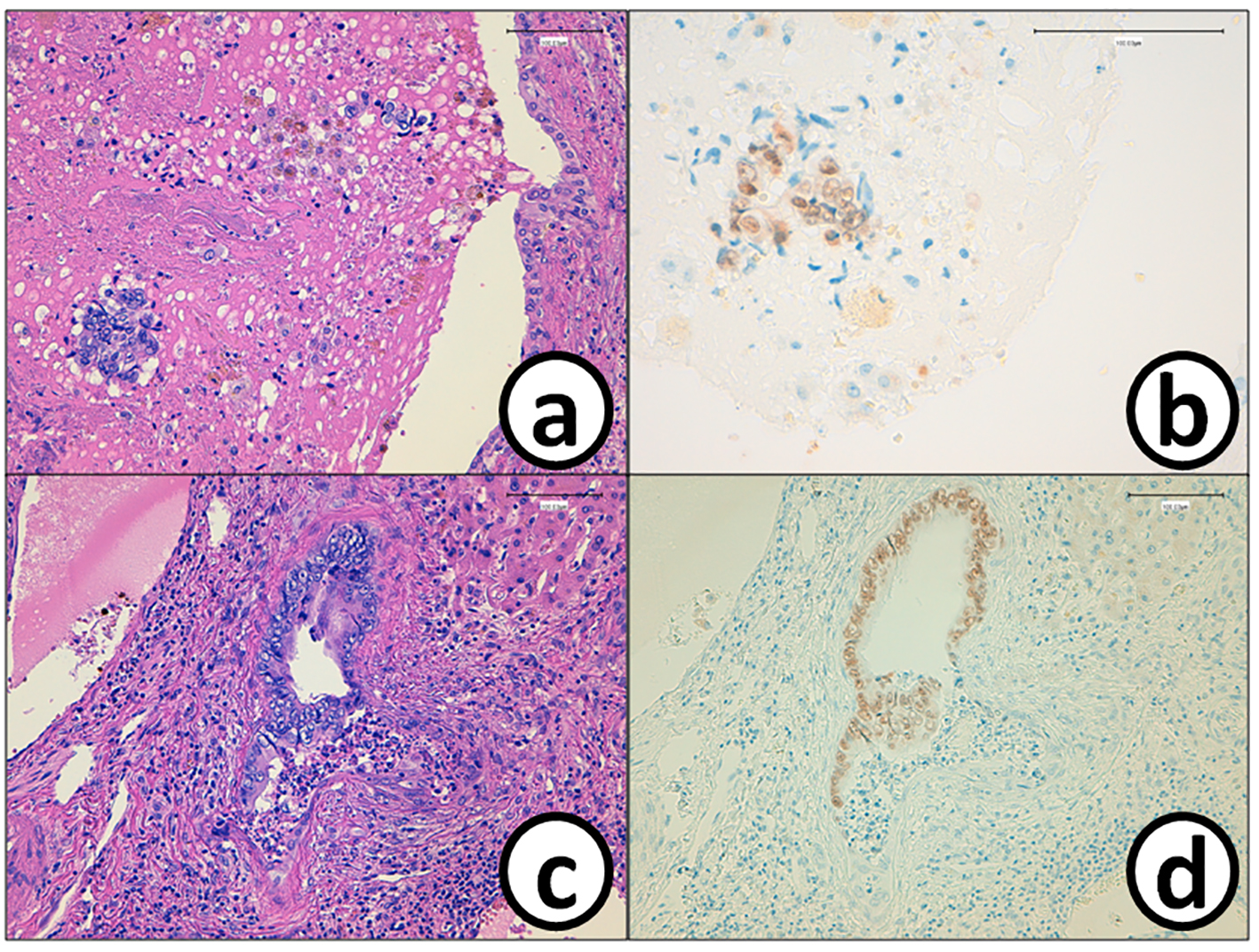 Click for large image | Figure 8. Cancer cells (a) in the tumor thrombus in the bile duct are positive for (b) CDX2. The epithelium of the bile duct is replaced by (c) metastatic colon cancer cells with (d) positive reaction of CDX2. Bars, 100 μm. (a, c) Hematoxylin and eosin stain. (b, d) Immunohistochemistry of CDX2. |
We thus finally diagnosed that the liver tumor was intrahepatic biliary metastasis of sigmoid colon adenocarcinoma. On diagnostic image examination, it is hard to detect biliary invasion and spread in a patient with liver metastasis of cancer. Knowledge of distinctive morphological and IHC features leads us to accurately diagnose such a rare intrahepatic biliary metastasis of colonic adenocarcinoma in routine pathological diagnostic procedures.
| Discussion | ▴Top |
In a few cases of liver metastasis of CRC, cancer cells spread along with intrabiliary ducts. We sometime misdiagnose such cases as intrahepatic cholangiocarcinoma. Previously the incidence of biliary metastasis of CRC in the liver was considered to be low. However, at present the frequency is not low and estimated to be 10-12% of the cases of liver metastasis of CRC [17, 18]. The clinicopathological characteristics of such cases include slow growing, low invasion, and relatively good prognosis [17, 18]. Clinical diagnosis of our case was initially hepatocellular carcinoma based on the findings of images’ investigation. However, IHC analysis using a variety of antibodies of the resected liver tumor revealed liver metastasis of CRC.
Although Okano et al [5] described utility of contrast CT for diagnosis of biliary metastasis of CRC by detecting enlargement of portal area, localized dilatation of intrahepatic bile duct, and wedge-shaped contrast area in the liver parenchyma, we do not frequently obtain such findings. Therefore, histopathological and IHC findings of the resected tumor are worthy for accurate diagnosis of intrabiliary metastasis of CRC [19-23].
Several mechanism(s) of hepatic intrabiliary metastasis of CRC have been proposed. They include vascular invasion via the lymph vessels, blood vessels, or biliary tract, and implantation of cancer cells to the bile duct epithelium via the bile stream [11]. It may also be possible that a peribiliary capillary plexus communicating with portal veins and hepatic arteries are the routes for metastatic cancer cells to seed along the bile duct epithelium [24]. However, in our case vascular invasion of the lymph and blood vessels was slight in the primary and metastatic tumors. Therefore, the other mechanism of metastasis via the bile stream [18, 25] is attractive. In this case, the cancer cells spreading along the basement membrane of the bile duct epithelium resulted in replacement of intrahepatic bile ducts.
Conclusion
Although liver metastatic lesions from CRC are usually regular and/or irregular round or nodular masses, the propensity for intrabiliary metastasis of CRC in the liver is now well recognized. Since bile duct invasions of CRC may be subtle or concealed by their response to chemotherapy, biliary metastasis should be carefully detected by high-resolution radiographic examination and pathological analysis with IHC.
Competing Interests
The authors declare that they have no competing interests.
| References | ▴Top |
- Taylor M, Forster J, Langer B, Taylor BR, Greig PD, Mahut C. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997;173(6):467-471.
doi - Yasui K, Hirai T, Kato T, Torii A, Uesaka K, Morimoto T, Kodera Y, et al. A new macroscopic classification predicts prognosis for patient with liver metastases from colorectal cancer. Ann Surg. 1997;226(5):582-586.
doi pubmed - Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353-1357.
doi pubmed - Wenzel DJ, Gaede JT, Wenzel LR. Case report. Intrabiliary colonic metastasis mimicking primary biliary neoplasia. AJR Am J Roentgenol. 2003;180(4):1029-1032.
doi pubmed - Okano K, Yamamoto J, Okabayashi T, Sugawara Y, Shimada K, Kosuge T, Yamasaki S, et al. CT imaging of intrabiliary growth of colorectal liver metastases: a comparison of pathological findings of resected specimens. Br J Radiol. 2002;75(894):497-501.
doi pubmed - Kawakatsu S, Kaneoka Y, Maeda A, Takayama Y, Fukami Y, Onoe S. Intrapancreatic bile duct metastasis from colon cancer after resection of liver metastasis with intrabiliary growth: a case report. World J Surg Oncol. 2015;13:254.
doi pubmed - Peungjesada S, Aloia TA, Kaur H, Marcal L, Choi H, Vauthey JN, Loyer EM. Intrabiliary growth of colorectal liver metastasis: spectrum of imaging findings and implications for surgical management. AJR Am J Roentgenol. 2013;201(4):W582-589.
doi pubmed - Coppola S, Zucchini N, Romano F, Bovo G, Gilardoni E, Nespoli L, Gianotti L. Colorectal liver metastasis with intrabiliary growth: case report and review of the literature. Int J Surg Pathol. 2014;22(3):272-279.
doi pubmed - Estrella JS, Othman ML, Taggart MW, Hamilton SR, Curley SA, Rashid A, Abraham SC. Intrabiliary growth of liver metastases: clinicopathologic features, prevalence, and outcome. Am J Surg Pathol. 2013;37(10):1571-1579.
doi pubmed - Riopel MA, Klimstra DS, Godellas CV, Blumgart LH, Westra WH. Intrabiliary growth of metastatic colonic adenocarcinoma: a pattern of intrahepatic spread easily confused with primary neoplasia of the biliary tract. Am J Surg Pathol. 1997;21(9):1030-1036.
doi pubmed - Yamao T, Hayashi H, Higashi T, Takeyama H, Kaida T, Nitta H, Hashimoto D, et al. Colon cancer metastasis mimicking intraductal papillary neoplasm of the extra-hepatic bile duct. Int J Surg Case Rep. 2015;10:91-93.
doi pubmed - Strauss AT, Clayton SB, Markow M, Mamel J. Colon Cancer Metastatic to the Biliary Tree. ACG Case Rep J. 2016;3(3):214-216.
doi pubmed - Povoski SP, Klimstra DS, Brown KT, Schwartz LH, Kurtz RC, Jarnagin WR, Fong Y, et al. Recognition of intrabiliary hepatic metastases from colorectal adenocarcinoma. HPB Surg. 2000;11(6):383-390; discussion 390-381.
- Natsume S, Kato T, Aoba T. Liver metastasis from sigmoid colon cancer with intrabiliary growth of mucinous cancer which had both intraepithelial and intraluminal components (in Japanese). J Jpn Biliary Assoc. 2013;27:746-751.
- Nihei Y, Morishima K, Miyakura Y, Sata N, Hironaka M, Yasuda Y. A case of liver metastasis with biliary invasion from colorectal cancer which was detected by diagnostic image techniqes (in Japanese). J Jpn Surg Assoc. 2010;71:2406-2410.
- Kawamura T, Morita T, Yamaguchi K, Okamura K, Horita S. A case of liver metastasis of colon cancer with intrabiliary growth and localized biliary dilatation (in Japanese). J Jpn Soc Coloproctol. 2009;62:165-168.
doi - Kubo M, Sakamoto M, Fukushima N, Yachida S, Nakanishi Y, Shimoda T, Yamamoto J, et al. Less aggressive features of colorectal cancer with liver metastases showing macroscopic intrabiliary extension. Pathol Int. 2002;52(8):514-518.
doi pubmed - Okano K, Yamamoto J, Moriya Y, Akasu T, Kosuge T, Sakamoto M, Hirohashi S. Macroscopic intrabiliary growth of liver metastases from colorectal cancer. Surgery. 1999;126(5):829-834.
doi - Bayrak R, Haltas H, Yenidunya S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: cytokeratin 7-/20+ phenotype is more specific than CDX2 antibody. Diagn Pathol. 2012;7:9.
doi pubmed - Sasaki A, Kawano K, Aramaki M, Nakashima K, Yoshida T, Kitano S. Immunohistochemical expression of cytokeratins in intrahepatic cholangiocarcinoma and metastatic adenocarcinoma of the liver. J Surg Oncol. 1999;70(2):103-108.
doi - Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer. 2002;38(6):758-763.
doi - Geller SA, Dhall D, Alsabeh R. Application of immunohistochemistry to liver and gastrointestinal neoplasms: liver, stomach, colon, and pancreas. Arch Pathol Lab Med. 2008;132(3):490-499.
pubmed - Kakar S, Gown AM, Goodman ZD, Ferrell LD. Best practices in diagnostic immunohistochemistry: hepatocellular carcinoma versus metastatic neoplasms. Arch Pathol Lab Med. 2007;131(11):1648-1654.
pubmed - Sahani DV, Kalva SP. Imaging the liver. Oncologist. 2004;9(4):385-397.
doi - Liu QY, Lin XF, Li HG, Gao M, Zhang WD. Tumors with macroscopic bile duct thrombi in non-HCC patients: dynamic multi-phase MSCT findings. World J Gastroenterol. 2012;18(11):1273-1278.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


