| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 9, Number 5-6, November 2018, pages 145-150
Role of Diffusion-Weighted Magnetic Resonance Imaging (DWMRI) in Assessment of Primary Penile Tumor Characteristics and Its Correlations With Inguinal Lymph Node Metastasis: A Prospective Study
Sasanka Kumar Baruaa, Pranab K. Kamana, Saumar Jyoti Baruaha, Rajeev T. Pa, Puskal K. Bagchia, Debanga Sarmaa, Yashasvi Singha, b
aDepartment of Urology, Gauhati Medical College Hospital, Guwahati, Assam, India
bCorresponding Author: Yashasvi Singh, Department of Urology , 3rd Floor, GMCH Complex, GMC Hostel Road, Bhangagarh, Guwahati 781032, Assam, India
Manuscript submitted August 25, 2018, accepted September 24, 2018
Short title: MRI in Carcinoma Penis
doi: https://doi.org/10.14740/wjon1138w
| Abstract | ▴Top |
Background: Penile cancer is a rare malignancy. The extent of lymph node (LN) metastasis is the most important prognostic factor in penile cancer. However, preoperative prediction of LN involvement in clinically non-palpable LN is still a challenge. In absence of a reliable biomarker, attempts are being made to validate imaging characteristics as a predictive tool. The aim of the present study is to assess the primary penile tumor characteristics with diffusion-weighted magnetic resonance imaging (DWMRI) and its correlations with inguinal LN status and tumor positivity in LN dissection specimen within normal sized LNs.
Methods: Twenty-six patients with carcinoma penis underwent DWMRI of penis and pelvis. The apparent diffusion coefficient (ADC) values of primary tumor were compared with histological characteristics. Inclusion criteria encompassed all cases of clinically non-palpable inguinal LN and normal sized LN on imaging. All palpable inguinal nodes with pelvic lymphadenopathies were excluded from this study.
Results: The primary tumor ADC ranged from 0.65 × 10-3 - 1.2 × 10-3 mm2/s (mean: 0.87 × 10-3 ± 0.11 × 10-3 mm2/s). In pT1 and pT3 tumors, mean ADC values were 0.86 × 10-3 ± 0.10 × 10-3 and 0.81 × 103 ± 0.09 × 103 mm2/s, respectively. The mean ADC values for grade 1, grade 2 and grade 3 were 0.89 × 10-3, 0.82 × 10-3 and 0.80 × 10-3 mm2/s, respectively. The ADC value of < 0.95 × 10-3 mm2/s was positively correlated with pathological LN presence within normal sized LN. With mean ADC value of 0.87 × 10-3 ± 0.11 × 10-3 mm2/s, sensitivity and positive predictive values for primary penile cancer were 100% and 84.61%, respectively. The mean ADC value for higher-grade and -stage tumor was low. The sensitivity and specificity of predicting LN metastasis by DWMRI were 87.22% and 80.90%, respectively.
Conclusion: ADC value of primary tumor can help in prediction of LN metastasis in carcinoma penis with clinically and radiologically normal groin.
Keywords: Carcinoma penis; Lymph node; DWMRI; ADC value
| Introduction | ▴Top |
Penile cancer is a rare entity which accounts for 0.4-0.6% of all malignant neoplasms among men [1]. Its expanse is more common in a developing country which is signified by the fact that it accounts for about 10% of all malignant disease burdens [2]. The presence and extent of lymph node (LN) metastasis is the most important prognostic factor in penile cancer which can be assessed by clinical examination, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI) or fluoro-deoxyglucose positron emission tomography. Diffusion-weighted MRI (DWMRI) is a functional imaging technique that depends upon Brownian motion of water which can calculate apparent diffusion coefficient (ADC) of tissue. ADC values provide functional information that distinguishes benign LNs from metastatic LNs even in similar size LNs reported as normal in radiological studies.
Aim
The aim of the study was to assess the primary penile tumor characteristics (size, site, invasion into tunica, corpus spongiosa, corpus cavernosa and urethra) with DWMRI and its correlations with normal sized inguinal LN (radiologically and clinically) characteristics such as size, shape, location, number, and internal nodal architecture and tumor positivity in LN dissection specimen.
| Patients and Methods | ▴Top |
This prospective study was conducted between March 2016 and December 2017 in the Department of Urology, GMCH Guwahati after obtaining clearance from the institutional ethical committee. A written consent was taken from all patients. The inclusion criteria comprised of all carcinoma penis with non-palpable inguinal LN and normal sized LN on imaging. The exclusion criteria included all palpable inguinal LN and pelvic lymphadenopathy. All patients with carcinoma penis were investigated with DWMRI of primary penile lesion, inguinal region and pelvis. All patients were investigated in flaccid state. After evaluation, all patients were treated with penectomy (partial/total) and inguinal LN dissection (unilateral/bilateral) with or without pelvic LN dissection (unilateral/bilateral). Any correlations between the findings of DWMRI of primary lesion and histopathological findings of primary tumor and dissected LNs were analyzed.
MRI was performed using a 1.5 T superconductive magnet MRI system with maximum gradient amplitude of 40 mT/m and a maximum slew rate of 135 mT/m/s along with a 16-channel-body array coil. For functional evaluation, DWMRI was performed in the transverse plane, with the following parameters: section thickness of 4 mm, intersection gap of 0.8 mm, three b values (0, 400 and 1,000 s/mm2) in three orthogonal directions with eight averages, 5,570/62, 975 Hz/pixel bandwidth, 300 × 300 mm2 field of view and 256 × 256 matrix. Presence of the tumor was defined as high signal intensity on diffusion-weighted imaging (DWI). The ADC value of the tumor and LNs was calculated in a circular region of interest for quantitative analysis. The correlation between variables was assessed using Pearson’s R coefficient and the test of significance was assessed with Chi-square, t-test, one- and two-way ANOVA. A P value of < 0.05 was considered significant. All these statistical analyses were done using SPSS version 21.0.
| Results | ▴Top |
This study included a total of 26 patients. Age groups ranged from 25 to 76 years (mean: 42.5 ± 12.29 years). Twelve (46.15%) patients presented with duration of > 12 months. Phimosis was associated with 17 (65.38%) patients. The most common site of involvement was distal third of penile shaft. ADC value of primary tumor ranged between 0.65 × 10-3 and 1.20 × 10-3 mm2/s (mean: 0.87 × 10-3 ± 0.11 × 10-3 mm2/s, 95% confidence interval (CI): 0.827 - 0.918). The pT1, pT2 and pT3 stages were found in 6 (23.07%), 9 (34.63%) and 11 (42.30%) patients respectively (Table 1).
 Click to view | Table 1. ADC Value and Stage of Primary Tumor |
The mean ADC values of pT1 and pT3 stage were 0.86 × 10-3 ± 0.10 × 10-3 mm2/s (95% CI: 0.77 - 0.95) and 0.81 × 10-3 ± 0.09 × 10-3 mm2/s (95% CI: 0.75 - 0.80), respectively (P = 0.045).
The numbers of patients in pathological grades 1, 2 and 3 were 18 (69.23%), 7 (26.92%) and 1 (3.85%), respectively, and their mean ADC values were 0.89 × 10-3, 0.82 × 10-3 and 0.80 × 10-3 mm2/s, respectively (Table 2).
 Click to view | Table 2. ADC Value and Histological Grade of Primary tumor |
When cutoff value of ADC was < 0.95 × 10-3 mm2/s, the sensitivity and positive predictive value were 100% and 84.61%, respectively.
The total number of LNs detected on DWMRI was 68 (34 each on both right and left sides) with mean size of 1.2 × 0.8 cm. Round and oval shaped LNs were seen in 76.47% and 23.53% cases, respectively. Heterogeneous and homogeneous LNs were observed in 58.82% and 41.18% cases, respectively.
On the right side, numbers of LNs extracted during surgery and tumor positivity (histopathological examination (HPE)) were 34 and 27 (79.41%), respectively, with ADC value ranging between 0.65 × 10-3 and 1.2 × 10-3 mm2/s (Table 3). Nevertheless, when ADC values were fixed to < 0.95 × 10-3 mm2/s, total number of positive biopsies was only 23 (P = 0.001).
 Click to view | Table 3. ADC Value and Histopathology of LN (Right Side) |
On the left side, numbers of LNs and tumor positivity were 34 and 24 (70.58%), respectively (Table 4), but when ADC values were fixed to < 0.95 × 10-3 mm2/s, number of tumor positivity on histology was 23 (P = 0.001).
 Click to view | Table 4. ADC Value and Histopathology of LN (Left Side) |
When cutoff value of ADC was < 0.95 × 10-3 mm2/s, its sensitivity, specificity, positive predictive value and negative predictive value of ADC values were 87.22%, 80.90%, 91.10% and 73.86%, respectively (P = 0.001). DWI showed an area under the receiver operating characteristic (ROC) curve (AUC) of 0.934 (standard error (SE): 0.042; 95% CI: 0.851 - 1) on the right side (Fig. 1a), whereas it showed an AUC of 0.885 (SE: 0.091; 95% CI: 0.706 - 1) on the left side (Fig. 1b).
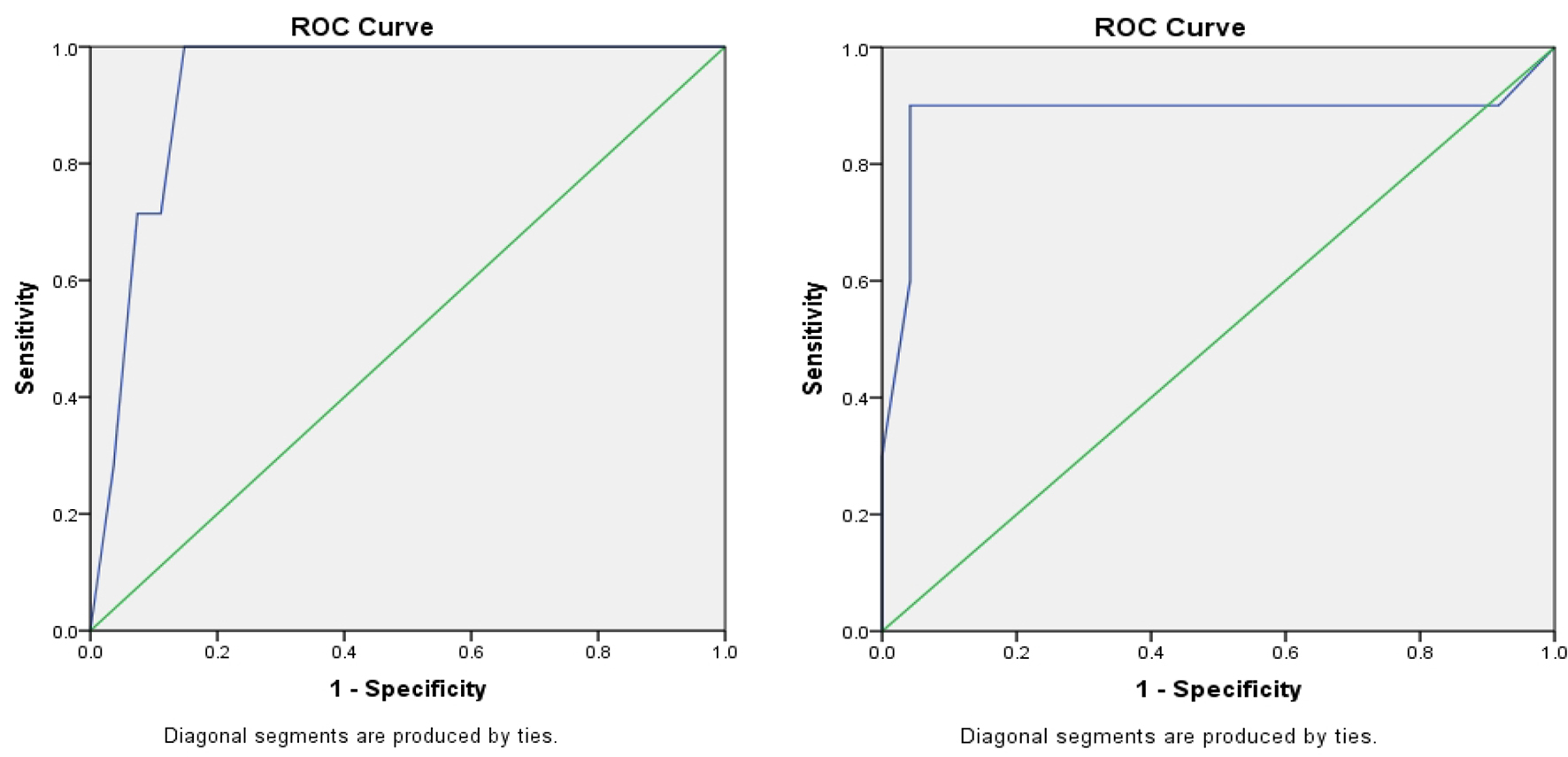 Click for large image | Figure 1. (a) ROC curve on the right side; (b) ROC curve on the left side. |
| Discussion | ▴Top |
Penile cancer is an aggressive and rare cancer and it usually affects older men. Gursel et al [3] along with Derrick and his team [4] showed that mean ages of occurrence were 58 and 55 years, respectively, though the tumor is not unusual in younger men. According to a study conducted by Dean and his team [5], 22% of patients were younger than 40 years and 7% were younger than 30 years. In our study, age ranged from 25 to 76 years (mean: 42.5 ± 12.29 years).
Neonatal circumcision has been authentically established as a prophylactic measure that virtually eliminates the occurrence of penile carcinoma. Phimosis is present in 25-75% of patients of carcinoma of penis [6]. In our study, 17 (65.38%) patients presented with phimosis.
In patients with penile cancer, both the primary tumor and the inguinal LNs are readily assessed by palpation. However, Horenblas and his team [7] found that physical examination incorrectly established the actual pathological stage in 26% of cases, with under and over staging of 10% and 16%, respectively. To bridge this gap of discrepancy between physical examination and tumor positivity in inguinal nodes, several studies are being carried out with ultrasonography and recently with MRI [8-10].
DWMRI is widely used in almost all disciplines for tumor detection, characterization and the evaluation of treatment response in patients with cancer [11]. Recent studies have described the usefulness of DWI for detecting malignant tumors of the liver, renal, prostate, colorectal and pancreas [12, 13]. DWI is a functional imaging technique that reflects changes in proton mobility caused by the alteration of tissue cellularity and the integrity of the cellular membrane, tortuosity of extracellular space and viscosity of fluids due to pathological processes [14-16]. The gradients are characterized by their b values, which express the amount of diffusion weighting [17, 18]. By performing DWI using different b values, quantitative analysis, namely, the calculation ADC values, is possible and the ADC values can be displayed as a parametric map (ADC map) [11]. It is observed that malignant tissues have higher signal intensity on DWI and lower ADC value.
Kobayashi et al [19] reported sensitivity rates > 90% for DWI for detecting bladder cancer with median ADC value of 0.86 × 10-3 mm2/s. Matsuki et al. [20] were able to visualize on DWI all 17 bladder cancers in 15 patients with sensitivity and positive predictive values of 100%. The mean ADC value of tumor was 1.18 × 10-3 mm2/s, which was significantly lower than those of surrounding structures. In our study, mean ADC value of primary penile tumor was 0.87 × 10-3 ± 0.11 × 10-3 mm2/s. The sensitivity and positive predictive value for detected primary penile cancer were 100% and 84.61%, respectively. Our observation is similar to the study conducted by aforementioned authors.
Takeuchi et al [21] recently reported that the mean ADC value of grade 3 bladder cancer was significantly lower than those of grade 1 and grade 2 tumors. In our study, the mean ADC values of grade 3, grade 2 and grade 1 penile cancer were 0.80 × 10-3, 0.82 × 10-3 and 0.89 × 10-3 mm2/s, respectively (P = 0.040), thus validating the abovementioned findings. Similarly, Kobayashi et al [19] reported that mean ADC values was lower in high-grade and high-stage tumors. In our study, mean ADC values of T3 and T1 stages were 0.817 × 10-3 ± 0.09 × 10-3 and 0.86 × 10-3 ± 0.10 × 10-3 mm2/s, respectively, which indicated that higher pT stage tumor has lower ADC value (P = 0.045).
The number of LNs detected with DWMRI preoperatively in our study was 68 (34 each on both right and left sides). The numbers of LNs positive on inguinal LN dissection (ILND) on right and left sides were 27 (79.41%) and 24 (70.59%), respectively, with ADC in the range of 0.65 × 10-3 - 1.2 × 10-3 mm2/s (mean ADC: 0.87 × 10-3 mm2/s). However, when the ADC value was fixed to < 0.95 × 10-3 mm2/s, numbers of positive histopathology for radiologically normal sized LNs on right and left sides were found to be 23 (100%) and 23 (95.83%), respectively (P = 0.001).
Squamous cell carcinoma of the penis metastasizes in a stepwise pattern. Skip metastases have rarely been reported. The clinically node-negative patients present a challenge for additional imaging as approximately 20% will harbor clinically undetectable metastases.
Lin et al [22] evaluated detection rate of pelvic LN metastasis in patients with cervical cancer on DWI. They further suggested that combination of size and relative ADC values were useful in detecting pelvic LN metastasis in these patients with a sensitivity of 83%.
Kim and his team [23] reported that the ADC values were significantly lower in the metastatic LN than in the non-metastatic LN of cervical cancer patients. They observed sensitivity and specificity to be 87% and 80% respectively with ADC value of 0.90 × 10-3 mm2/s.
In our study, mean ADC value of primary tumor which was deemed to harbor metastasis was 0.87 × 10-3 mm2/s and sensitivity, specificity, positive predictive value and negative predictive value were 87.22%, 80.90%, 91.10% and 73.86%, respectively. Our study has congruent observation with aforementioned authors.
On cross-sectional imaging, normal LNs usually appear homogeneous, oval- or cigar-shaped with well-defined borders (Table 5). According to van den Brekel et al [24] and Imhof et al [25], round-shaped nodes were more likely to harbor metastases. In our study, round-shaped metastatic LNs were found 76.47% and 64.71% on right and left sides, respectively. We have not found any significant correlations among these matched variables.
 Click to view | Table 5. Sizes of LNs as reported in MRI |
Conclusions
It is evident from our study that the metastatic LNs in the inguinal region can be precisely predicted by DWMRI by correlating ADC value of primary tumor even when the sizes of LNs are within normal limit. We perceived a positive correlation between ADC values of primary tumor and LN positivity for metastasis even in normal sized LNs. We have not found any significant correlations between the ADC values of primary tumor, internal nodal architecture and shape of LN. This may be due to small sample size in our study. Therefore, further long-term multi-institutional studies with larger sample size are required for its validation.
| References | ▴Top |
- Vatanasapt V, Martin N, Sriplung H, Chindavijak K, Sontipong S, Sriamporn H, Parkin DM, et al. Cancer incidence in Thailand, 1988-1991. Cancer Epidemiol Biomarkers Prev. 1995;4(5):475-483.
pubmed - Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27(2):141-150.
doi pubmed - Gursel EO, Georgountzos C, Uson AC, Melicow MM, Veenema RJ. Penile cancer. Urology. 1973;1(6):569-578.
doi - Derrick FC, Jr., Lynch KM, Jr., Kretkowski RC, Yarbrough WJ. Epidermoid carcinoma of the penis: computer analysis of 87 cases. J Urol. 1973;110(3):303-305.
doi - Dean AL Jr. Epithelioma of the penis. J Urol. 1935;33:252-283.
doi - Pettaway CA, Crook JM, Pagliaro LC. Tumours of the Penis. Wein AJ, editor. Campbell - Walsh Urology, 11th edition. Philadelphia: Elsevier publishers; 2016. p. 847.
- Horenblas S, Van Tinteren H, Delemarre JF, Moonen LM, Lustig V, Kroger R. Squamous cell carcinoma of the penis: accuracy of tumor, nodes and metastasis classification system, and role of lymphangiography, computerized tomography scan and fine needle aspiration cytology. J Urol. 1991;146(5):1279-1283.
doi - Vapnek JM, Hricak H, Carroll PR. Recent advances in imaging studies for staging of penile and urethral carcinoma. Urol Clin North Am. 1992;19(2):257-266.
pubmed - Vossough A, Pretorius ES, Siegelman ES, Ramchandani P, Banner MP. Magnetic resonance imaging of the penis. Abdom Imaging. 2002;27(6):640-659.
doi pubmed - Hanchanale V, Yeo L, Subedi N, Smith J, Wah T, Harnden P, Bhattarai S, et al. The accuracy of magnetic resonance imaging (MRI) in predicting the invasion of the tunica albuginea and the urethra during the primary staging of penile cancer. BJU Int. 2016;117(3):439-443.
doi pubmed - Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188(6):1622-1635.
doi pubmed - Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246(3):812-822.
doi pubmed - Xu PJ, Yan FH, Wang JH, Lin J, Ji Y. Added value of breathhold diffusion-weighted MRI in detection of small hepatocellular carcinoma lesions compared with dynamic contrast-enhanced MRI alone using receiver operating characteristic curve analysis. J Magn Reson Imaging. 2009;29(2):341-349.
doi pubmed - Koyama T, Togashi K. Functional MR imaging of the female pelvis. J Magn Reson Imaging. 2007;25(6):1101-1112.
doi pubmed - Kilickesmez O, Bayramoglu S, Inci E, Cimilli T, Kayhan A. Quantitative diffusion-weighted magnetic resonance imaging of normal and diseased uterine zones. Acta Radiol. 2009;50(3):340-347.
doi pubmed - Whittaker CS, Coady A, Culver L, Rustin G, Padwick M, Padhani AR. Diffusion-weighted MR imaging of female pelvic tumors: a pictorial review. Radiographics. 2009;29(3):759-774; discussion 774-758.
- Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, Scheenen TW, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261(1):46-66.
doi pubmed - Hafeez S, Huddart R. Advances in bladder cancer imaging. BMC Med. 2013;11:104.
doi pubmed - Kobayashi S, Koga F, Yoshida S, Masuda H, Ishii C, Tanaka H, Komai Y, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. 2011;21(10):2178-2186.
doi pubmed - Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol. 2007;17(1):201-204.
doi pubmed - Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, Suzuki K, et al. Urinary bladder cancer: diffusion-weighted MR imaging - accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009;251(1):112-121.
doi pubmed - Lin G, Ho KC, Wang JJ, Ng KK, Wai YY, Chen YT, Chang CJ, et al. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008;28(1):128-135.
doi pubmed - Kim JK, Kim KA, Park BW, Kim N, Cho KS. Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: early experience. J Magn Reson Imaging. 2008;28(3):714-719.
doi pubmed - van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, Meyer CJ, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177(2):379-384.
doi pubmed - Imhof H, Czerny C, Dirisamer A. Head and neck imaging with MDCT. Eur J Radiol. 2003;45(Suppl 1):S23-31.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


