| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 9, Number 5-6, November 2018, pages 151-155
EGFR Mutation Detection and Its Association With Clinicopathological Characters of Lung Cancer Patients
Priyanka Gaura, Sandeep Bhattacharyaa, d, Surya Kantb, R.A.S. Kushwahab, Gaurav Singhc, Sarika Pandeyb
aDepartment of Physiology, King George’s Medical University, UP, Lucknow-226010, Uttar Pradesh, India
bDepartment of Respiratory Medicine, King George’s Medical University, UP, Lucknow-226010, Uttar Pradesh, India
cCSIR-Institute of Genomics and Integrative Biology, Delhi, India
dCorresponding Author: Sandeep Bhattacharya, Department of Physiology, King George’s Medical University, UP, Lucknow-226010, Uttar Pradesh, India
Manuscript submitted October 19, 2018, accepted November 6, 2018
Short title: EGFR Mutation and Lung Cancer
doi: https://doi.org/10.14740/wjon1167
| Abstract | ▴Top |
Background: Lung cancer is the most common type of cancer worldwide with an estimation of 1.82 million new cancer cases diagnosed; and it is the leading cause of cancer-related deaths. Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase identified as being highly expressed in cancer cells including lung cancers. The aim of the study is to determine the EGFR mutation status in non-small cell lung cancer (NSCLC) patients to investigate the association between the EGFR mutation status and clinicopathological characters of patients.
Methods: The tissue samples of the lung cancer patients were collected bronchoscopically. The EGFR mutations of 70 NSCLC patients were determined by the immunohistochemistry (IHC).
Results: EGFR mutations were present in 24 cases (34.29%), including 19 (79.13%) cases of exon 19 and five (20.83%) cases of exon 21 mutation. EGFR mutations were frequently associated with adenocarcinoma and non-smoker. Statistically significant association of EGFR mutations with adenocarcinoma subtypes and non-smokers was found (P < 0.05); and no significant association of EGFR mutation with the age of the patient (P = 0.4647) and the stage (P = 0.4578) of the tumor was found. When we compared between these two mutations, no significant association with age (P=0.614) and smoking status (P=0.127) was found in this study.
Conclusions: EGFR mutations were significantly associated with female sex, non-smoker and adenocarcinoma subtypes. The analysis of EGFR mutation by the IHC method is a potentially useful tool to guide clinicians for personalized treatment of NSCLC patients of adenocarcinoma subtype.
Keywords: Lung cancer; EGFR; Immunohistochemistry; Non-small cell lung carcinomas
| Introduction | ▴Top |
Lung cancer is the most common type of cancer worldwide with an estimation of 1.82 million new cancer cases diagnosed. It is the leading cause of cancer-related deaths [1]. Lung cancers are mainly classified into two major types including small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), mainly consists of adenocarcinoma and squamous cell carcinoma [2]. Tobacco smoking is a widely recognized risk factor for lung cancer in squamous cell carcinoma and SCLC, but smoke exposure seems to be a less potent oncogenic factor for adenocarcinoma. Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase. It has been identified as highly expressed in cancer cells including lung cancer also [3]. EGFR is a transmembrane protein having an extracellular ligand-binding domain, a transmembrane domain, an intracellular tyrosine kinase (TK) domain and a regulatory region [4]. After binding to the ligands specific tyrosine residues of the intracellular domain, it becomes autophosphorylated and results in the initiation of the intracellular signaling cascade including the Ras/Raf/MAPK, JAK/STAT and PI3K-Akt pathways which leads to the cell proliferation, cell differentiation, angiogenesis, metastasis and antiapoptosis [5]. The discovery of oncogenic driver mutations in the EGFR gene of exons 18-21 and approval of agents which target against these molecular drivers have revolutionized the management of NSCLC [6]. Small molecule tyrosine kinase inhibitors (TKIs) such as gefitinib, erlotinib, and afatinib have been targeted against the EGFR, significantly improved the response rates and progression-free survival when used in patients with activating mutations of the EGFR gene [7]. Gefitinib is an orally active EGFR TK inhibitor, which has been widely used in clinical trials and approved for the treatment of advanced NSCLC [8-10]. Since surgical intervention, platinum-based chemotherapy and radiotherapy were the principal available therapeutic options available for the treatment of lung cancer, but with the description of EGFR mutations in lung adenocarcinoma in the past decade and the response of these tumors to TKIs such as gefitinib and erlotinib, a new hope in making a significant difference in the survival of such patients has arisen [11, 12]. In this study we determine the EGFR mutation status in NSCLC patients to investigate the association between the EGFR mutation and clinicopathological characters of patient.
| Materials and Methods | ▴Top |
The study was conducted at the Department of Respiratory Medicine, King George’s Medical University, Lucknow, India. The study was approved by the ethics committee of the corresponding institute and all the subjects gave their written consent. This study included only lung cancer patients; and subjects having other disorders such as chronic obstructive pulmonary disease (COPD), asthma, tuberculosis, interstitial lung disease and other malignancies were excluded from the study. Tissue samples of the lung cancer subjects were collected bronchoscopically for the detection of EGFR mutations. The EGFR mutation was detected by immunohistochemistry (IHC) method.
Formalin-fixed, paraffin-embedded tissue sections were cut into 4-µm-thick sequential sections. After deparaffinization and rehydration, sections were boiled in citrate buffer (0.01 M, pH 6.0) for antigen retrieval. Sections were then incubated with 3% H2O2 and 5% serum to block endogenous peroxidase activity and non-specific binding. Two primary antibodies (delE746-A750 mutation specific monoclonal antibody (6B6) and L858R mutation specific monoclonal antibody (43B2); Cell Signaling Technology, Danvers, MA, USA) were used for detection of EGFR mutation. The sections were then incubated with biotinylated secondary antibodies and visualized by DAB. Counterstaining was carried out with hematoxylin. The sections were dehydrated in alcohol and mounted with DPX.
The IHC staining score was based on the staining intensity and percentage positivity (0-100%) of cells in the membrane and/or cytoplasm of tumor cells. Four grades were employed: 0, 1+, 2+, and 3+. 0 means no staining; 1+ means faint membrane and/or cytoplasmic staining in less than 10% positive cells; 2+ means moderate membrane and/or cytoplasmic staining in greater than 10% and less than 50% cells; 3+ means strong membrane and/or cytoplasmic staining more than 50% cells positive. 0 and 1+ scores were considered as negative; whereas 2+ and 3+ were considered as positive cases.
Data were analyzed using Graph pad prism statistical software (version 5). Descriptive data were presented as mean, standard deviation (SD) or as percentages. Comparison between the groups was done using the Chi-square/Fishers exact test for categorical variables. P value < 0.05 was defined as being statistically significant.
| Results | ▴Top |
The demographic and clinical characteristics of lung cancer patients and controls are shown in Table 1. The mean age of the lung cancer patients was 53.67 years old; Out of the 70 lung cancer patients 52 (80.4%) patients were male, and 18 (19.6%) were female. In the present study the highest percentage of the lung cancer patients were non-smokers and comprises 39 (55%) of the patients. Most of the lung cancer patients were of stage III/IV which consist of 69 (98.57%) of patients. Fifty-eight (89.23%) of the patients have the clinical symptoms of cough followed by chest pain and breathlessness comprising 53 (81.54%).
 Click to view | Table 1. Demographic and Clinical Profile of Lung Cancer Patients |
In this study EGFR mutation was found in 34.29% (24/70) of NSCLC cases. Figure 1 represents the E746-A750 and L858R positive and negative mutation. The mean age of mutation positive and negative cases are (55.58 ± 9.39) and (53.67 ± 10.93). No statistically significant difference between age of the lung cancer patient and EGFR mutation status was found (P = 0.4647). Out of female included in the study, 50% (9/18) females showed EGFR mutation positive compared to 28.85% (15/52) of males and the association was statistically non significant (P = 0.614) (Table 2). Out of the non-smokers included in the study, 46.15% (18/39) showed EGFR mutations positive compared to 28.57% (2/7) of the smoker and 16.7% (4/24) ex-smokers groups which shows statistically significant difference (P < 0.05) (Table 2). There is no statistically significant association between stage of the tumor and EGFR mutation status observed (P = 0.457).
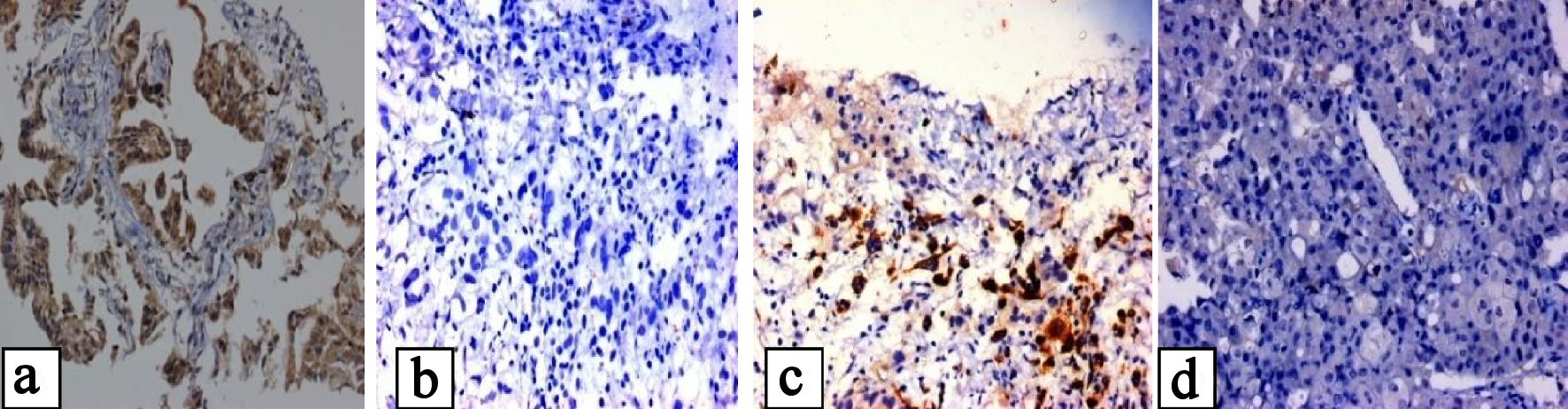 Click for large image | Figure 1. (a, b) Showing positive and negative EGFR mutation of E746-A750 in exon 19, and (c, d) showing positive and negative EGFR mutation of L858R mutation in exon 21. |
 Click to view | Table 2. Clinical Characteristics of Lung Cancer Patients Having Positive and Negative EGFR Mutations |
The study shows that exon 21 mutation was found in 85.8% (6/7) of male and 14.2% (1/7) of females while mutation in exon 19 was seen in 57.8% (11/19) in males and 42.1(8/11) of females. No statistically significant difference between type of EGFR mutation, gender (P = 0.1362), smoking status (P = 0.6404) and histological subtypes (P = 0.4438) of the tumors was observed (Table 3).
 Click to view | Table 3. Relationship Between the Type of EGFR Mutation and Clinicopathological Characters of Lung Cancer Patients |
It has been observed from the study that deletion mutations in exon 19 was the most common and seen in 79.17 % (19/24) of the cases (Fig. 2). Out of all the mutation positive cases, 20.83% (5/24) had L858R point mutations of exon 21 (Fig. 2).
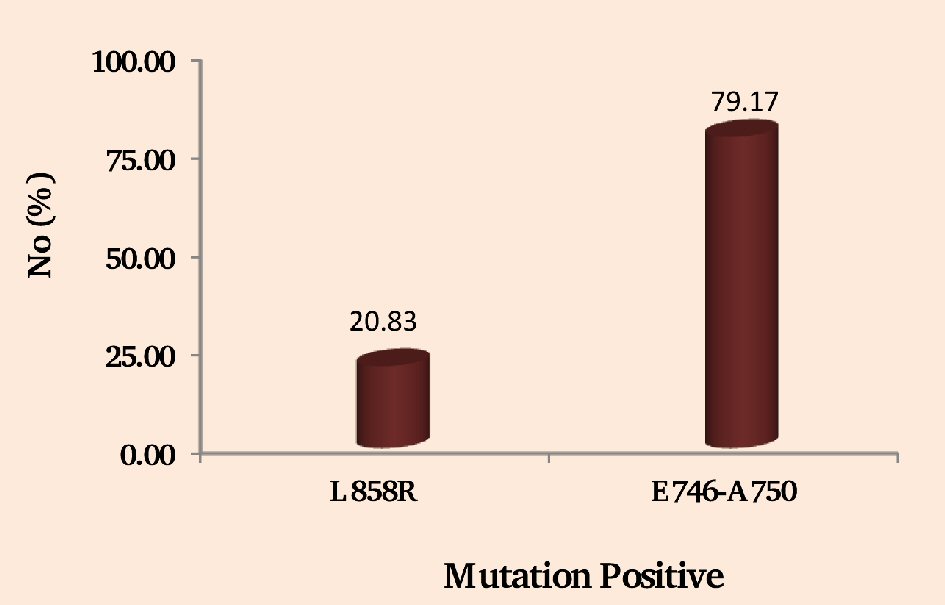 Click for large image | Figure 2. Showing the NSCLC patients having positive EGFR mutations of exon 21 (L858R) and exon 19 (E746-A750). |
| Discussion | ▴Top |
EGFR mutation status is the most valuable indicator for the screening of NSCLC patients. It has been reported that mutations in exon 19 were the most common of all EGFR mutations. Higher prevalence of EGFR mutations is reported in females, non-smokers and patients with adenocarcinoma by the previous study [13]. Lung adenocarcinoma from non-smoker and female gender are more likely to contain mutations in the EGFR gene and therefore may show a better response to gefitinib. In the present study the EGFR mutations were present in highest percentage in the male sex and in non-smokers.
Since no correlation between the presence of EGFR mutations and the stage of disease was found in this study hence EGFR mutation is an early event that plays an important role in the pathogenesis of lung adenocarcinoma.
It has been reported from the previous study that EGFR mutations is found in 40-55% of adenocarcinoma [14, 15]. The prevalence of EGFR gene mutations in NSCLC cases has been reported from 3% to 40% by previous studies worldwide [11-15]. In this study EGFR mutation is found in 36 % of NSCLC patients.
It has been reported from the previous studies that in frame deletions in exon 19 was present in 26-79% while the point mutations in exon 21 (L858R) was seen in 13-47% [16]. It have been reported from the previous studies that exon 19 deletions was more susceptible to gefitinib than tumors with exon 21 point mutations [17]. In the present study in frame deletion of exon 19 mutation was the most common EGFR mutations seen in 79.17 % of the cases, point mutations in exon 21 (L858R) observed in 20.83% cases which show similarity with the previous studies. Mutations in the EGFR gene have been reported to be associated with response of lung cancer patients to TKI such as gefitinib [9, 18]. Although the recent advances are applicable for the management of advanced NSCLC, the cure rate remains still low; hence further molecular investigations are required for the development of the new treatment strategies to improve the prognosis of lung cancer patients. It has been shown by several studies that the EGFR mutations are predictive factors of response to EGFR-TKI treatment. The testing of NSCLC patients with adenocarcinoma subtype of the lung for selection of specific therapy is standard of care in clinical practice. Most of the lung cancer patients where only small biopsies or cytological material are available that may benefit from molecular testing to determine the choice of drugs for target therapy [19].
Conclusions
In frame deletion of exon 19 mutation was the most common EGFR mutation, and EGFR mutations were significantly associated with female sex, non-smoker and adenocarcinoma subtypes. The analysis of EGFR mutation by the IHC method is a potentially useful tool to guide clinicians for personalized treatment of NSCLC patients of adenocarcinoma subtype. EGFR mutation status is the most valuable indicator for the screening of NSCLC patients for TKI therapy. The detection of EGFR mutations in NSCLC patients is helpful in selection of targeted therapy.
Acknowledgments
We would like to thank to the faculty and staff of the Department of Respiratory Medicine, King George’s Medical University, Lucknow for providing samples. The authors also would like to thank all the patients and their families for their cooperation and participation.
Financial Support
None.
Conflict of Interest
None.
| References | ▴Top |
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer, Lyon, 2014
- Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. Histological typing of lung and pleural tumors. Berlin: Springer; 1999. p. 1-156.
doi pubmed - Rusch V, Baselga J, Cordon-Cardo C, Orazem J, Zaman M, Hoda S, McIntosh J, et al. Differential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lung. Cancer Res. 1993;53(10 Suppl):2379-2385.
pubmed - Cohen S, Carpenter G, King L, Jr. Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980;255(10):4834-4842.
pubmed - Ciardiello F, De Vita F, Orditura M, Tortora G. The role of EGFR inhibitors in nonsmall cell lung cancer. Curr Opin Oncol. 2004;16(2):130-135.
doi pubmed - Singh N, Bal A, Aggarwal AN, Das A, Behera D. Clinical outcomes in non-small-cell lung cancer in relation to expression of predictive and prognostic biomarkers. Future Oncol. 2010;6(5):741-767.
doi pubmed - Hirsch FR, Janne PA, Eberhardt WE, Cappuzzo F, Thatcher N, Pirker R, Choy H, et al. Epidermal growth factor receptor inhibition in lung cancer: status 2012. J Thorac Oncol. 2013;8(3):373-384.
doi pubmed - Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD, Jr., Morse D, Abraham S, et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212-1218.
doi pubmed - Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
doi pubmed - Barker AJ, Gibson KH, Grundy W, Godfrey AA, Barlow JJ, Healy MP, Woodburn JR, et al. Studies leading to the identification of ZD1839 (IRESSA): an orally active, selective epidermal growth factor receptor tyrosine kinase inhibitor targeted to the treatment of cancer. Bioorg Med Chem Lett. 2001;11(14):1911-1914.
doi - Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497-1500.
doi pubmed - Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129-2139.
doi pubmed - Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64(24):8919-8923.
doi pubmed - Huang SF, Liu HP, Li LH, Ku YC, Fu YN, Tsai HY, Chen YT, et al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res. 2004;10(24):8195-8203.
doi pubmed - Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba, II, Fong KM, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339-346.
doi pubmed - Sholl LM, Xiao Y, Joshi V, Yeap BY, Cioffredi LA, Jackman DM, Lee C, et al. EGFR mutation is a better predictor of response to tyrosine kinase inhibitors in non-small cell lung carcinoma than FISH, CISH, and immunohistochemistry. Am J Clin Pathol. 2010;133(6):922-934.
doi pubmed - Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, Hatooka S, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23(11):2513-2520.
doi pubmed - Cappuzzo F, Gregorc V, Rossi E, Cancellieri A, Magrini E, Paties CT, Ceresoli G, et al. Gefitinib in pretreated non-small-cell lung cancer (NSCLC): analysis of efficacy and correlation with HER2 and epidermal growth factor receptor expression in locally advanced or metastatic NSCLC. J Clin Oncol. 2003;21(14):2658-2663.
doi pubmed - Jiang G, Fan C, Zhang X, Dong Q, Wang L, Liu Y, Dai S, et al. Ascertaining an appropriate diagnostic algorithm using EGFR mutation-specific antibodies to detect EGFR status in non-small-cell lung cancer. PLoS One. 2013;8(3):e59183.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


