| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Letter to the Editor
Volume 11, Number 1, February 2020, pages 41-43
The Emergence of Epstein-Barr Virus-Related Diffuse Large B-Cell Lymphoma With Mogamulizumab
Lan Wanga, Miriam Bargouta, Jane Date Honb, Bassam Yaghmourc, Ann Mohrbachera, George Yaghmoura, d
aDivision of Hematology, University of Southern California, Norris Comprehensive Cancer Center, Los Angeles, CA 90033, USA
bDivision of Pathology, University of Southern California, Los Angeles, CA 90033, USA
cPulmonary, Critical Care, Keck School of Medicine of University of Southern California, Los Angeles, CA 90033, USA
dCorresponding Author: George Yaghmour, Division of Hematology, University of Southern California, 1441 Eastlake Ave, Los Angeles, CA 90033, USA
Manuscript submitted December 23, 2019, accepted January 15, 2020
Short title: EBV-Related DLBCL With Mogamulizumab
doi: https://doi.org/10.14740/wjon1255
Angioimmunoblastic T-cell lymphoma (AITL) is one of the most common types of T cell lymphomas, representing approximately 15-20% of all cases [1]. The CC chemokine receptor 4 (CCR4) is a transmembrane chemokine receptor that plays an important role in the homing and migration of T-cells to the skin, and its expression has been seen in patients with cutaneous T cell lymphomas (CTCL) and adult T-cell lymphomas (ATL). Mogamulizumab is the first humanized monoclonal antibody directed against CCR4, and is currently Food and Drug Administration (FDA)-approved in the USA for the treatment of mycosis fungoides and Sezary syndrome [2, 3]. Mogamulizumab exerts its antitumor activity through antibody-dependent cellular cytotoxicity (ADCC) against CCR4-positive malignant T cells and through downregulation of regulatory T cells (Tregs) to enhance the immune response [4, 5]. Mogamulizumab has shown promising results as both monotherapy and in combination chemotherapy in the treatment of T-cell lymphomas, including AITL, in the relapsed/refractory setting through several phase I and II studies [6]. Here, we report a case of Epstein-Barr virus (EBV)-related diffuse large B-cell lymphoma (DLBCL) that developed after the use of mogamulizumab for the treatment of relapsed AITL.
A 73-year-old female with a past medical history of eosinophilic fasciitis, presented with left axillary and left inguinal lymphadenopathy that was biopsied showing ill-defined nodular and diffuse aggregates (cluster of differentiation (CD)3+, CD4+, CD10+, PD1+, and CD30 with 10% partial expression) admixed with larger immunoblasts and prominent blood vessels, consistent with AITL. The patient was referred to our center from outside facility; we could not obtain a picture of the pathology at initial diagnosis. The patient initially opted for a trial of alternative therapy but re-presented 7 months later with extensive cutaneous disease in her arms, face, neck, and anterior chest, worsening lymphadenopathy (Fig. 1a) and fatigue. She still did not want to start conventional chemotherapy, so she was initiated on the anti-CD30 antibody drug conjugate, brentuximab, along with palliative radiation for symptomatic management. In spite of initial near clearance of all her skin lesions and most of her lymphadenopathy on brentuximab (Fig. 1b), after nine doses over 7 months, subsequent positron emission tomography-computed tomography (PET-CT) showed evidence of progression with worsening lymphadenopathy (Fig. 1c). The patient refused to have a repeated biopsy and after tumor board discussion, the patient was then started on mogamulizumab, with resolution of her skin lesions and cervical lymphadenopathy. However, repeat PET-CT after 10 doses of the mogamulizumab, showed mixed response but with the development of new hypermetabolic lymph nodes consistent with progression of disease (Fig. 1d), so the patient was referred for a clinical trial. However, prior to the first course of the trial treatment, a repeat lymph node biopsy of the left axillary lymph node showed evidence of EBV + DLBCL, without definitive evidence of her previous AITL (Fig. 2). Given the new pathology findings, the patient was started on bendamustine with rituximab, with the anti-CD79a monoclonal antibody, polatuzumab.
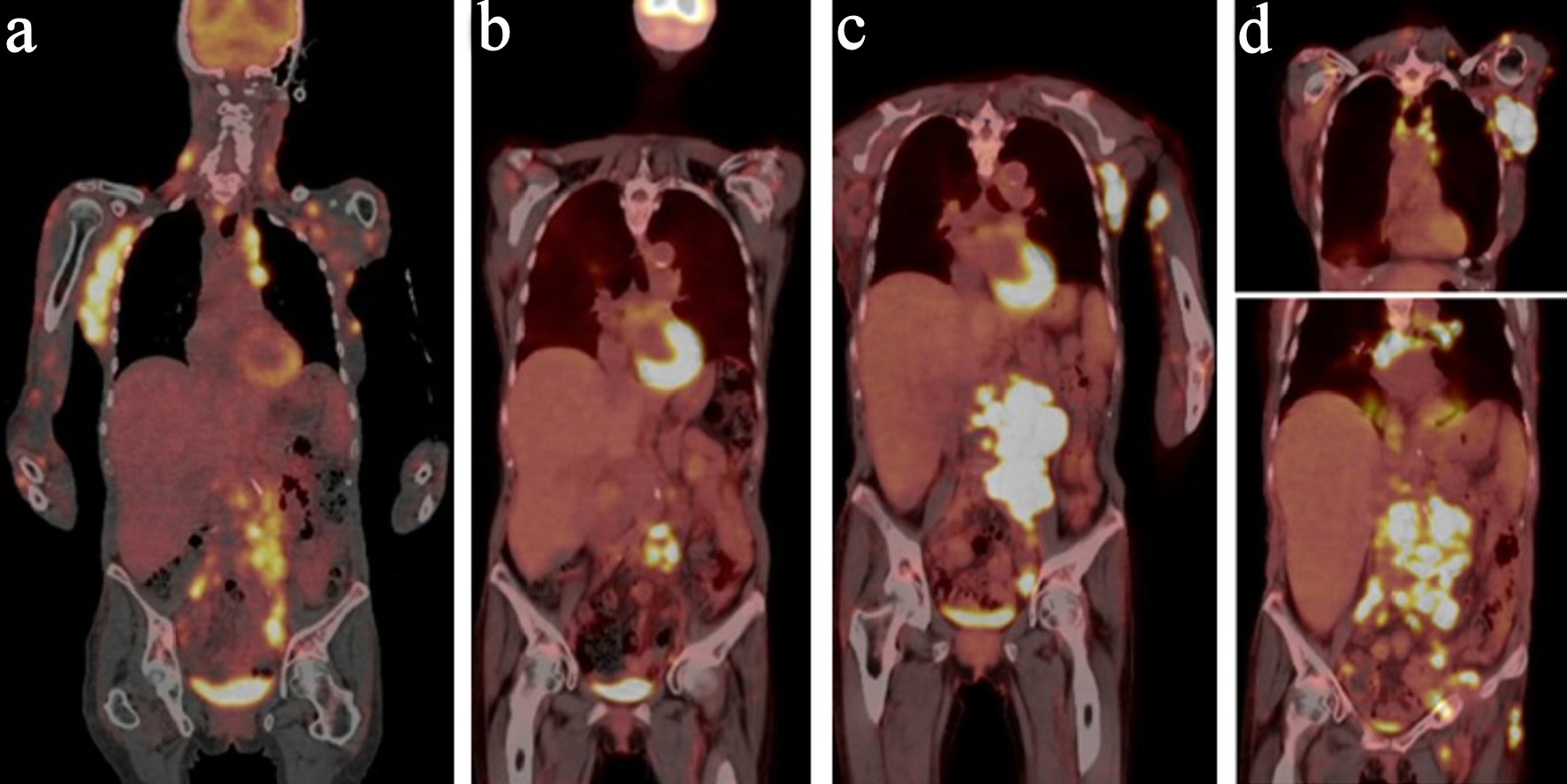 Click for large image | Figure 1. PET-CT panel with initial PET-CT, PET-CT with progression in February, 2019, PET-CT in May, 2019 and PET in July, 2019. (a) PET-CT at initial diagnosis. (b) PET-CT after brentuximab. (c) PET-CT with progression on brentuximab. (d) PET-CT with progression on mogamulizumab. PET-CT: positron emission tomography-computed tomography. |
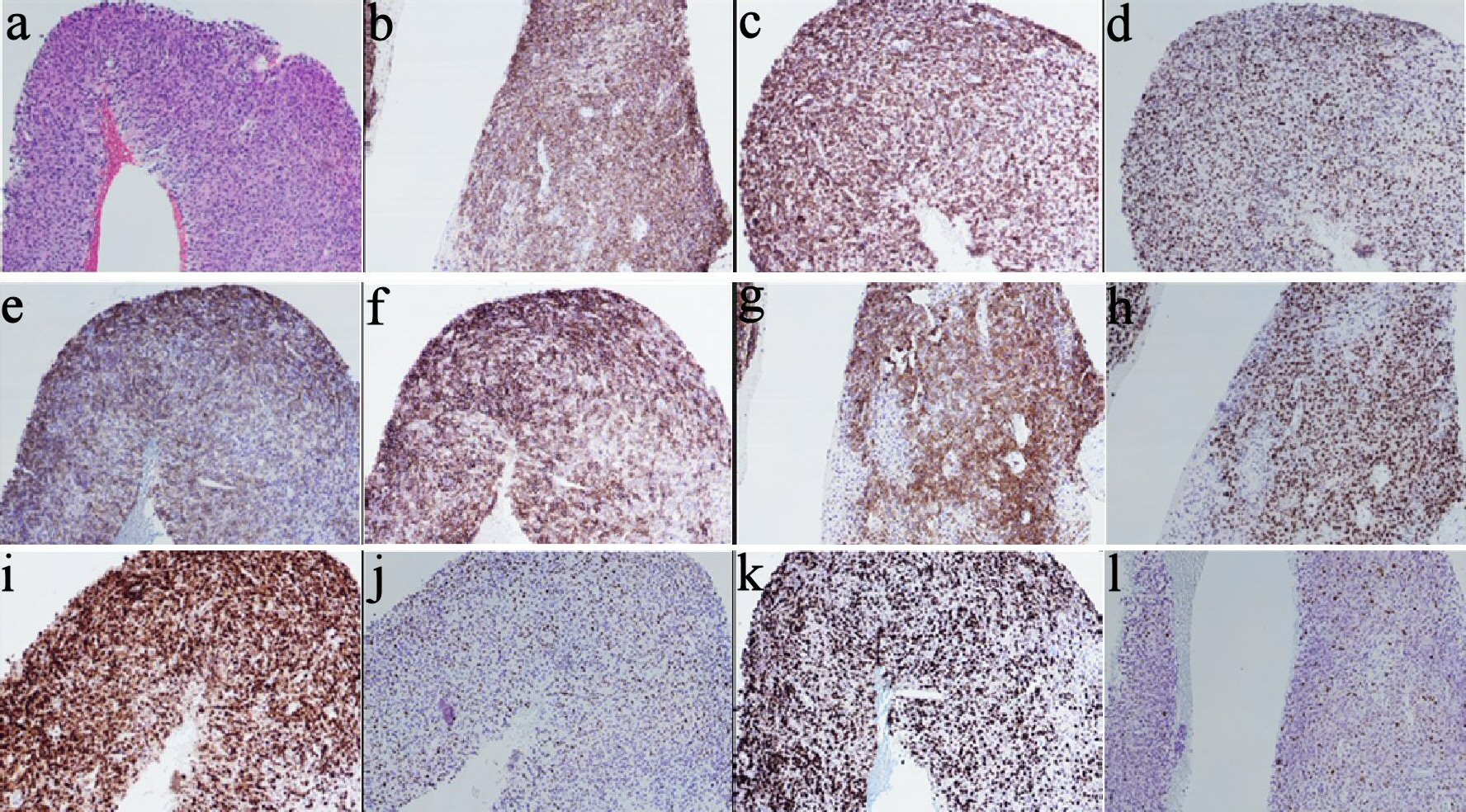 Click for large image | Figure 2. Pathology images/panel with EBV+ DLBCL. (a) H&E stain showing large lymphoma cells infiltrating the tissue in sheets (× 10). (b) CD20 IHC (× 10). (c) BCL2 IHC (× 10). (d) BCL6 IHC (× 10). (e) CD21 IHC (× 10). (f) CD23 IHC (× 10). (g) CD30 IHC (× 10). (h) OCT2 IHC (× 10). (i) MUM1 IHC (× 10). (j) Twenty to thirty percent of cells positive for MYC IHC (× 10). (k) High proliferation index (80-90%) by Ki-67 IHC (× 10). (l) Patchy positive EBER-ISH (× 10). EBV: Epstein-Barr virus; DLBCL: diffuse large B-cell lymphoma; H&E: hematoxylin and eosin stain; IHC: immunohistochemistry; ISH: in situ hybridization; CD: cluster of differentiation. |
Although mogamulizumab has shown efficacy in patients with AITL, it also interferes with normal immune defenses relying on chemotaxis of T cells to areas of infection. The monoclonal antibody has been reported to cause immunopathologies such as graft-versus-host disease, especially of the skin. The immunomodulatory effects, along with the prolonged lymphopenia from mogamulizumab therapy, have been proposed to cause reactivation of viral infections such as cytomegalovirus (CMV), hepatitis B and EBV [1, 7-9]. The reactivation of EBV may, in turn, cause B-cell lymphoproliferative disorders as well as the emergence of EBV-positive DLBCL, as in our patient (Table 1, [8, 10]).
 Click to view | Table 1. Summary of All Reported Patients Treated with Mogamulizumab and Developed EBV-Associated DLBCL |
This case implies that although mogamulizumab has efficacy against T cell lymphomas, the off-target effects of mogamulizumab on T cells normally involved in immune surveillance against virally infected cells can lead to the selective emergence of EBV-positive DLBCL. Administration of this medication requires careful monitoring and surveillance to detect emerging infections and lymphomas.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
I George Yaghmour disclose Takeda, Jazz, and Astellas Board Speaker, Incyte and Jazz Advisory board. The authors do not have any conflicts of interest.
Informed Consent
Not applicable.
Author Contributions
Lan Wang, Miriam Bargout, Jane Date Hon, Bassam Yaghmour, Ann Mohrbacher, and George Yaghmour performed the research and wrote the paper. Ann Mohrbacher and George Yaghmour supervised the study.
| References | ▴Top |
- Yabe M, Dogan A, Horwitz SM, Moskowitz AJ. Angioimmunoblastic T-Cell Lymphoma. Cancer Treat Res. 2019;176:99-126.
doi pubmed - Kasamon YL, Chen H, de Claro RA, Nie L, Ye J, Blumenthal GM, Farrell AT, et al. FDA Approval summary: mogamulizumab-kpkc for mycosis fungoides and Sezary syndrome. Clin Cancer Res. 2019;25(24):7275-7280.
doi pubmed - Ni X, Jorgensen JL, Goswami M, Challagundla P, Decker WK, Kim YH, Duvic MA. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sezary syndrome. Clin Cancer Res. 2015;21(2):274-285.
doi pubmed - Murai K, Hiroyuki H, Sato A, Yasuro M, Mori Y, Sakuma T. Mogamulizumab monotherapy in the treatment of relapsed/refractory peripheral T-cell lymphoma or cutaneous T-cell lymphoma patients in single-institution experience. Blood. 2017;130:(Supplement 1):5179.
doi - Yoshie O. Expression of CCR4 in adult T-cell leukemia. Leuk Lymphoma. 2005;46(2):185-190.
doi pubmed - Ishida T, Utsunomiya A, Jo T, Yamamoto K, Kato K, Yoshida S, Takemoto S, et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. 2017;108(10):2022-2029.
doi pubmed - Ishii Y, Itabashi M, Numata A, Yamamoto W, Motohashi K, Hagihara M, Matsumoto K, et al. Cytomegalovirus pneumonia after anti-CC-chemokine receptor 4 monoclonal antibody (Mogamulizumab) therapy in an angioimmunoblastic T-cell lymphoma patient. Intern Med. 2016;55(6):673-675.
doi pubmed - Kamachi K, Shindo T, Miyahara M, Kitaura K, Akashi M, Shin IT, Suzuki R, et al. Epstein-Barr virus-related diffuse large B-cell lymphoma in mogamulizumab-treated adult T-cell leukemia with incomplete T-cell reconstitution. Int J Hematol. 2019;109(2):221-227.
doi pubmed - Nakagawa M, Schmitz R, Xiao W, Goldman CK, Xu W, Yang Y, Yu X, et al. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med. 2014;211(13):2497-2505.
doi pubmed - Tanaka H, Aoki H, Sugita Y, Shimizu R, Kiko K, Mochida H, Suzuki Y. Development of epstein-barr virus-related primary diffuse large B-cell lymphoma of the central nervous system in a patient with peripheral T-cell lymphoma, not otherwise specified after mogamulizumab treatment. Intern Med. 2017;56(20):2759-2763.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


