| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 11, Number 3, June 2020, pages 112-115
First Report of Severe Acute Graft-Versus-Host Disease After Allogeneic Stem Cell Transplant in a Patient With Myelodysplastic Syndrome Treated With Atezolizumab: Literature Review
Mindy Hsiaoa, Sergei Tatishchevb, Tarek Khedroa, Bassam Yaghmourc, Casey O’Connella, George Yaghmoura, d
aDivision of Hematology, University of Southern California, Norris Comprehensive Cancer Center, Los Angeles, CA 90033, USA
bDepartment of Medicine, Department of Pathology, University of Southern California, Norris Comprehensive Cancer Center, Los Angeles, CA 90033, USA
cDivision of Pulmonary, Critical Care and Sleep Medicine, University of Southern California, Keck School of Medicine of USC, Los Angeles, CA 90033, USA
dCorresponding Author: George Yaghmour, University of Southern California, NTT3467, 1441 Eastlake Avenue, Los Angeles, CA 90033, USA
Manuscript submitted February 6, 2020, accepted March 18, 2020
Short title: Acute GVHD After Transplant in MDS Patient
doi: https://doi.org/10.14740/wjon1263
| Abstract | ▴Top |
Checkpoint inhibitors have become a widely used and available immunotherapy option for treating a variety of malignancies, including hematological malignancies. Patients receiving these therapies may go on to receive a curative allogeneic hematopoietic stem cell transplant (allo-HSCT). This presents a clinical challenge as the safety and efficacy of HSCT is not well reported in this subset of patients and residual programmed death-ligand 1 inhibition could potentially enhance allogeneic T-cell responses, improving the graft-versus-tumor effect, but also increasing the incidence and severity of immune complications such as graft-versus-host disease (GVHD). Here, this report includes a detailed literature review summarizing all available data on HSCT outcomes in the setting of using checkpoint inhibitor therapy pre-transplant. Moreover, we report a case of acute GVHD after allo-HSCT in a patient with high-risk myelodysplastic syndrome who received prior atezolizumab therapy, highlighting the importance of further research into this specific population in order to improve transplant-related outcomes.
Keywords: GVHD; Allogeneic stem cell transplant; Immunotherapy; Checkpoint inhibitors; Atezolizumab
| Introduction | ▴Top |
Immunotherapy, using tumor-targeted monoclonal antibodies or checkpoint inhibitors (CPIs), has become a widely used and available option for treating a variety of malignancies. Prior research has demonstrated that tumors expressing programmed death-ligand 1 (PD-L1) are able to inactivate cytotoxic T lymphocytes (CTLs) through engagement with inhibitory programmed death receptor-1 (PD-1) that are present on T cells leading to a reduction in cytokine production and proliferation of T cells. The process of disease progression is likely related to the up-regulation of PD-L1 expression allowing many cancers to evade the host immune response [1]. Atezolizumab, a PD-L1 monoclonal antibody, has been shown to be efficacious in treating solid tumors (i.e. melanoma, colon cancer, renal cell carcinoma) and most recently, when used as front-line therapy in metastatic non-small cell lung carcinoma, lead to significantly improved progression-free survival and overall survival [2]. Atezolizumab is currently being studied in many hematologic malignancies as well, though its overall efficacy is unknown. As there have been promising results in using both PD-L1 and PD-1 monoclonal antibodies to treat hematologic malignancies in early clinical trials, patients receiving these therapies may go on to receive a curative allogeneic hematopoietic stem cell transplant (allo-HSCT). This presents a clinical challenge as the safety and efficacy of HSCT is not well known in this subset of patients and residual PD-L1 inhibition could potentially enhance allogeneic T-cell responses, improving the graft-versus-tumor effect, but also increasing the incidence and severity of immune complications such as graft-versus-host disease (GVHD) [3]. This article illustrates a case of patient receiving atezolizumab prior to allo-HSCT with subsequent immune complications which has never been reported.
Moreover, we aim to highlight the importance of considering a washout period between last treatment and the time of transplant, applying T-cell depleted methods (post-transplant cytoxan), and considering reduced intensity conditioning regimen to prevent GVHD and improve outcomes.
| Case Report | ▴Top |
A 69-year-old Korean man with a history of chronic obstructive pulmonary disease, diabetes and prostate cancer treated with prostatectomy and radiation in 2008 was diagnosed with myelodysplastic syndrome, refractory anemia with excess blast (RAEB type1) in July 2017 when he presented with pancytopenia. The bone marrow biopsy obtained at that time showed 6-9% blasts by morphology with normal conventional cytogenetics and fluorescence in situ hybridization (FISH) studies. Myeloid mutation panel showed multiple mutations (positive for SRSF2, RUNX1, ASXL1, STAG2 and a BRAF VUS). His Revised International Prognostic Scoring System (R-IPSS) score was estimated as high risk (4.95) and the patient was initiated on azacitidine treatment in September 2017. Unfortunately, he progressed in February 2018 and thus was enrolled in a clinical trial studying guadecitabine and atezolizumab as combination therapy with clinical course complicated by multiple hospital admissions for neutropenic fever. Subsequent bone marrow biopsy after four cycles showed response with < 5% blasts by morphology and the dose of guadecitabine was reduced to prevent neutropenic fever complications; however, he remained pancytopenic. After five cycles of treatment, given 840 mg per cycle, with a total of seven atezolizumab doses (5,880 mg total), he received an allogeneic matched-unrelated donor peripheral blood stem cell transplant 10/12 permissive DPB1 mismatch and reduced intensity conditioning with fludarabine and melphalan 27 days from his last dose of atezolizumab. Plasma levels of atezolizumab were not assessed during the study, pre-, peri-, or post-transplant. He received tacrolimus on D-1 and methotrexate on D+1, D+3, D+6 and D+11 for GVHD prophylaxis. His post-transplant course was complicated by presumed engraftment syndrome empirically treated with steroids on D+9, acute kidney injury which resolved with fluids (however several tacrolimus doses were held), encephalopathy likely exacerbated by steroids, and biopsy-proven acute GVHD of the skin, gastrointestinal tract, and liver (liver stage 3, skin stage 2, gastrointestinal (GI) stage 3) that became steroid-refractory (Figs. 1 and 2 of GI and liver biopsies, respectively). After aggressive therapy with budesonide, high-dose steroids, ruxolitinib and beta-human chorionic gonadotropin (HCG), the patient succumbed to the complications of his disease after being found to have disseminated mucormycosis.
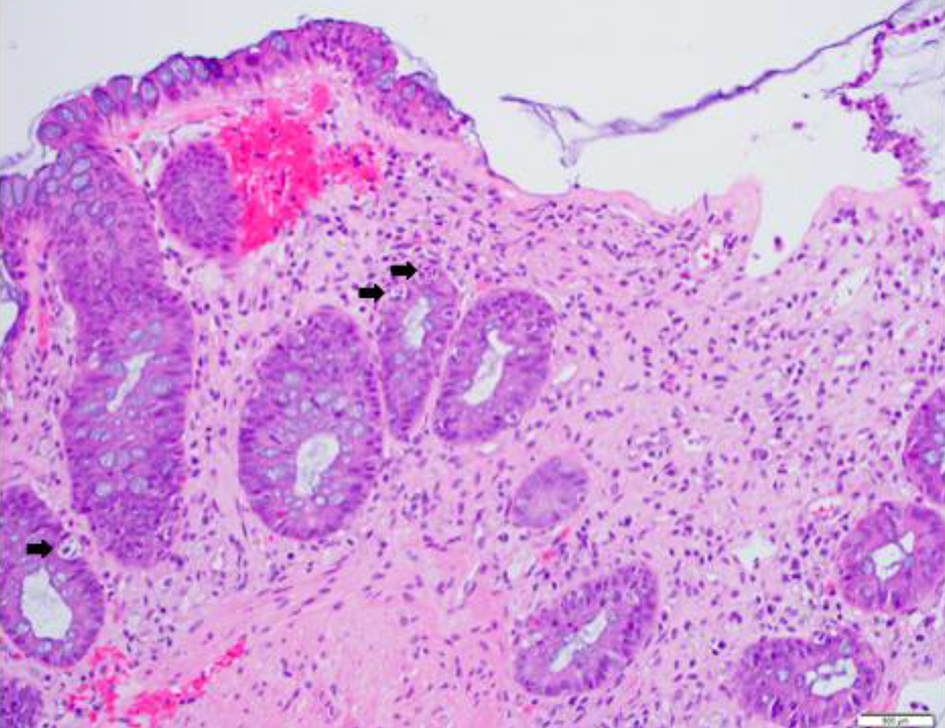 Click for large image | Figure 1. Colonic tissue with features of graft-versus-host disease. Histologic section of a colonic biopsy shows extensive crypt cell apoptosis, crypt destruction and dropout with focal mucosal necrosis and epithelial denudation. Black arrows indicate most prominent single-cell crypt apoptosis (hematoxylin-eosin, original magnification, × 200). |
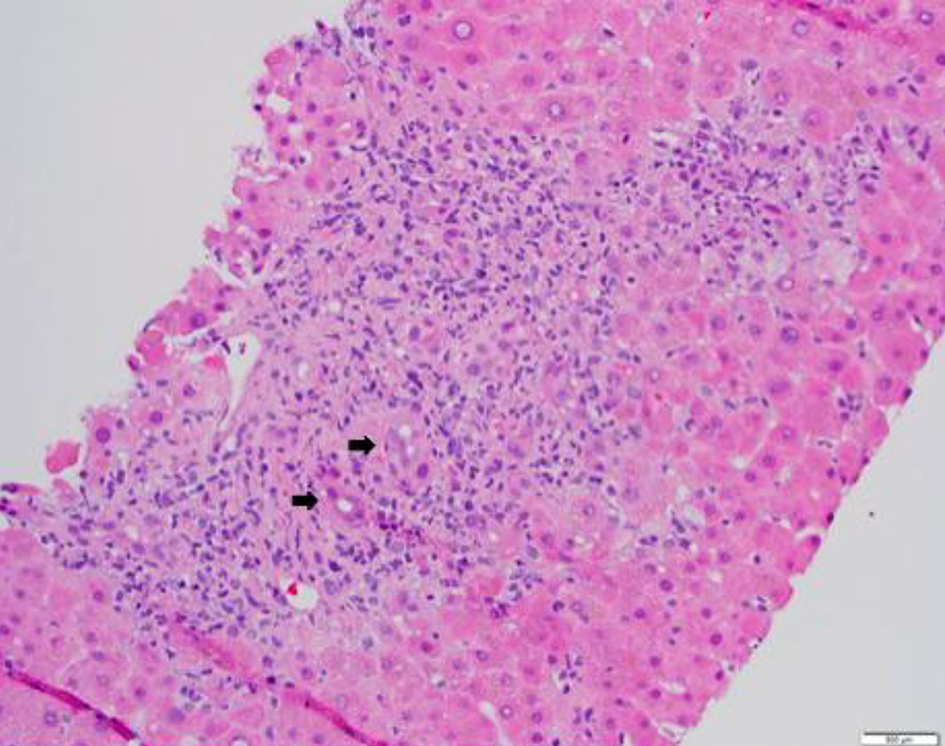 Click for large image | Figure 2. Liver tissue with features of graft-versus-host disease. Histologic section of a core liver biopsy shows mixed portal inflammatory infiltrates composed of neutrophils, lymphocytes and occasional plasma cells. There is extensive bile duct injury and interface hepatitis. Black arrows indicate damaged bile ducts (hematoxylin-eosin, original magnification, × 200). |
| Discussion | ▴Top |
In the era of immunotherapy, where CPIs are increasingly being used to treat aggressive hematologic malignancies, more data on the outcomes of these patients that eventually undergo allo-HSCT are becoming available. An increased risk of GVHD with prior exposure to CPIs has been observed and the FDA has subsequently issued label warnings for the use of allo-HSCT after previous PD-1 blockade [4]. Less is known about PD-L1 inhibitors, specifically atezolizumab, though theoretically, the same complications would occur. GVHD, unfortunately, remains a major cause of morbidity and mortality following allo-HSCT. Despite current prophylactic strategies, approximately 40-60% of all HSCT recipients may still develop acute GVHD, though currently numbers have been improving, and 20-50% will develop chronic GVHD [5]. At this time, corticosteroids are the only standard initial treatment and response rates are as low as 40-60% though many alternative therapies are currently being studied. The increased utilization of CPIs in hematologic malignancies, specifically in Hodgkin’s lymphoma, is associated with increased incidence and severity of GVHD in these patients that proceed to allo-HSCT, with acute GVHD rates remaining high at 56% and mortality risk at 11%, higher than the reported 8-10% in the general HSCT population [5]. The etiology is thought to be due to residual PD-1 inhibition peri- and post-HSCT leading to enhancement of allogeneic T-cell responses which may augment graft versus tumor (GVT) effect but also increase the incidence of GVHD which has been demonstrated in animal models [6].
In a review of the literature, several studies have confirmed these findings. Most recently, Ijaz et al [7] performed a meta-analysis evaluating GVHD risk pre- and post-allo-HSCT that included 107 patients who received CPI before undergoing allo-HSCT. Ninety-one patients received nivolumab for a median of 4 to 11 cycles, 11 patients received pembrolizumab for eight cycles and eight patients received four cycles of ipilimumab. High rates of hyperacute (7%), acute (56%) and chronic (29%) GVHD were observed, and of the 20 reported deaths, 60% were GVHD-related with an overall mortality risk from GVHD at 11%, higher than reported incidences; however, statistical significance was not established. In comparing the studies with shorter median intervals (30 days or less) with longer intervals (40 days or more), the incidence of GVHD was 60% compared with 70% respectively; however, the limited number of patients in each group was noted [7].
Most other abstracts and studies [8-11] that have evaluated CPIs prior to HSCT concluded that CPI use prior to HSCT may contribute to the increased risk of acute GVHD and although Schoch et al [8] did not note any deaths from GVHD, they did state that patients that received their last CPI dose within 90 days of transplant may have an increased risk of GVHD (Supplementary Material 1, www.wjon.org). Of note, only three studies documented the GVHD prophylaxis that was used, which is imperative to discuss, as the specific therapy of GVHD prophylaxis may be important in this setting. Historically, T-cell depletion (TCD) was used as a technique to reduce GVHD combined with positive CD34 stem cell selection and “mega dose” infusion; however, issues with immune reconstitution and increased rates of infections and relapse were observed [12, 13]. Subsequently, high-dose, single agent post-transplant cyclophosphamide (PTCY) was found to be effective as GVHD prophylaxis and is now standardly used, especially in the setting of haplo-identical [14]. More recently, Ikegawa et al investigated the mechanism of PD-1 blockade in GVHD and how PTCY may impact this process in murine models [15]. They found that inhibited PD-1 signaling led to increased and enhanced expansion of CD4+ T cells and unfortunately, Treg cells were unable to support the expansion due to increased apoptosis, resulting in deficiencies in the immune recovery, allowing for development of severe GVHD. Using PTCY on day +3 after transplantation in mice, it was noted that CD4+ T cell populations were decreased with increased Treg populations in the first 2 weeks compared with mice that did not receive PTCY, allowing for a well-balanced immune reconstitution and less GVHD [15]. This study provided insight in how PTCY may be beneficial as a method of GVHD prophylaxis in patients who received prior PD-1 blockade.
Nieto et al [16] performed a prospective study of 12 patients with various lymphoproliferative neoplasms who received prior anti-PD-1 therapy and were to undergo allo-HSCT from a matched related or unrelated donor. The study aimed to evaluate the differences in immune response during allo-HSCT between those that had received previous nivolumab therapy and those that did not in addition to evaluating the role of PTCY as GVHD prophylaxis in this setting. The six patients who were exposed to nivolumab prior to transplant received a median of 8.5 doses with a median time of 84 days between last dose and transplant. Three out of the six patients received standard GVHD prophylaxis with tacrolimus/sirolimus and all of them developed grade 3-4 acute GVHD compared with the other three patients who received PTCY as GVHD prophylaxis. In the control groups, one from each group had developed grade 2 acute GVHD. However no other patients developed grade 3-4 disease. Furthermore, nivolumab levels were tested on day +21 after HSCT in conjunction with an evaluation of changes in T-cell repertoire by flow cytometry. Investigators found that the residual nivolumab can be detected as late as 56 days after allo-HSCT and in those that received nivolumab and standard GVHD prophylaxis, the T-cell repertoire was altered suggesting immune activation was present and thus explains the development of acute GVHD in these patients when compared with those that did not receive nivolumab previously. In addition, those that received nivolumab and PTCY had a similar T-cell repertoire to those that had not received nivolumab previously and PTCY for GVHD prophylaxis suggesting that previous nivolumab exposure has little effect on PTCY. Both nivolumab-exposed groups were compared as well and a higher CD4+/CD8+ ratio, higher proportion of CD4+ and CD8+ effector memory T cells, lower percentage of IFN-γ-producing T cells with an attenuated Th1 response and decreased proportion of classical monocyte were seen in the PTCY group which may explain why acute GVHD was not observed in this group.
Although PTCY for GVHD prophylaxis is more standardly used in haplo-identical transplant, its role in matched unrelated and related allo-HSCT is not as ubiquitous [17, 18]. PTCY in this setting, however, has been studied with findings suggesting PTCY after MAC is suitable for single-agent prophylaxis against GVHD [14] and specifically in the setting of CPI use prior to allo-HSCT, this approach may be preferable as demonstrated in Nieto et al [16].
In summary, CPI therapy has become increasingly more widespread as its efficacy in treating various malignancies is now better established; however, it has been associated with increased incidence and severity of GVHD. The time between the last dose of CPI and allo-HSCT, in addition to the use of PTCY for GVHD prophylaxis, may be important factors affecting the outcomes of these patients. Our case is the first reported case of acute GVHD after allo-HSCT in a patient who received prior atezolizumab therapy, highlighting the importance of further research in this specific population in order to improve transplant-related outcomes.
| Supplementary Material | ▴Top |
Suppl 1. A Summary of Abstracts and Studies That Have Evaluated Checkpoint Inhibitors Prior to Allogeneic Hematopoietic Stem Cell Transplant.
Acknowledgments
None to declare.
Financial Disclosure
George Yaghmour declares Board speaker for Jazz, Takeda, and Astella. Casey O’Connell is the PI of guadecitabine and atezolizumab trial.
Conflict of Interest
The authors do not have any conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Mindy Hsiao, Tarek Khedro, Bassam Yaghmour and George Yaghmour performed the research, data analysis, and wrote the manuscript. Sergei Tatishchev provided and analyzed the pathology slides. George Yaghmour supervised the study.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580-6587.
doi pubmed - Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301.
doi pubmed - Saha A, Aoyama K, Taylor PA, Koehn BH, Veenstra RG, Panoskaltsis-Mortari A, Munn DH, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122(17):3062-3073.
doi pubmed - Kasamon YL, de Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R. FDA Approval Summary: Nivolumab for the Treatment of Relapsed or Progressive Classical Hodgkin Lymphoma. Oncologist. 2017;22(5):585-591.
doi pubmed - Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296-307.
doi pubmed - Bekoz H, Karadurmus N, Paydas S, Turker A, Toptas T, Firatli Tuglular T, Sonmez M, et al. Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience. Ann Oncol. 2017;28(10):2496-2502.
doi pubmed - Ijaz A, Khan AY, Malik SU, Faridi W, Fraz MA, Usman M, Tariq MJ, et al. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transplant. 2019;25(1):94-99.
doi pubmed - Schoch LK, Borrello I, Fuchs EJ, Bolanos-Meade J, Huo JS, Gojo I, et al. Checkpoint inhibitor therapy and graft versus host disease in allogeneic bone marrow transplant recipients of haploidentical and matched products with post-transplant cyclophosphamide. Blood. 2016;128(22):4571.
doi - Armand P, Zinzani PL, Collins GP, Cohen JB, Halwani AS, Carlo-Stella C, et al. Outcomes of allogeneic hematopoietic stem cell transplantation (HSCT) after treatment with nivolumab for relapsed/refractory Hodgkin lymphoma. Blood. 2016;128(22):3502.
doi - Covut F, Pinto R, Cooper BW, Tomlinson B, Metheny L, Malek E, Lazarus HM, et al. Nivolumab before and after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2017;52(7):1054-1056.
doi pubmed - El Cheikh J, Massoud R, Abudalle I, Haffar B, Mahfouz R, Kharfan-Dabaja MA, Jisr T, et al. Nivolumab salvage therapy before or after allogeneic stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transplant. 2017;52(7):1074-1077.
doi pubmed - Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186-1193.
doi pubmed - Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447-3454.
doi pubmed - Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, Huff CA, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224-3230.
doi pubmed - Ikegawa S, Meguri Y, Kondo T, Sugiura H, Sando Y, Nakamura M, Iwamoto M, et al. PTCy ameliorates GVHD by restoring regulatory and effector T-cell homeostasis in recipients with PD-1 blockade. Blood Adv. 2019;3(23):4081-4094.
doi pubmed - Nieto JC, Roldan E, Jimenez I, Fox L, Carabia J, Orti G, et al. Graft-Versus-Host disease (GVHD) prophylaxis with post-transplant cyclophosphamide (PTCY) induces a more tolerant immune response after allogeneic hematopoietic cell transplantation (allo-HCT) in patients previously exposed to nivolumab. Blood. 2018;132(Suppl 1):3402.
doi - Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650.
doi pubmed - Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, Armand P, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


