| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 11, Number 3, June 2020, pages 106-111
Clinical Characteristics and Outcome Trends of Adjuvant Anthracycline and Taxane Regimen for Early Stage Breast Cancer
Ashok K. Vaida, h, Aseem Khuranab, Devender Sharmac, Dheeraj Gautamd, Jyoti Wadhwac, Rajiv Agarwale, Kanchan Kaure, Jyoti Aroraf, Kush Guptag
aMedanta Cancer Institute, Medanta-The Medicity, Gurugram, India
bMedical Oncology, Sarvodaya Multispecialty and Cancer Hospital, Hisar, India
cMedical Oncology, Medanta Cancer Institute, Medanta-The Medicity, Gurugram, India
dDepartment of Histopathology, Medanta Cancer Institute, Medanta-The Medicity, Gurugram, India
eBreast Services, Medanta Cancer Institute, Medanta-The Medicity, Gurugram, India
fRadiology and Imaging, Medanta Cancer Institute, Medanta-The Medicity, Gurugram, India
gCatalyst Clinical Services Pvt. Ltd, New Delhi, India
hCorresponding Author: Ashok K. Vaid, Medanta Cancer Institute, Medanta-The Medicity, Gurugram, India
Manuscript submitted April 14, 2020, accepted April 27, 2020
Short title: Adjuvant Chemotherapy for Early Breast Cancer
doi: https://doi.org/10.14740/wjon1284
| Abstract | ▴Top |
Background: The anthracycline and taxane-based chemotherapy treatment regimen remains the gold standard for treatment of early stage breast cancer. However, studies examining the effectiveness and use of this treatment regimen in Indian context are limited. This study examined patients treated with anthracycline and taxane-based chemotherapy at a tertiary care cancer center in India.
Methods: Patients with confirmed early stage breast cancer who had undergone primary breast surgery followed by treatment with anthracycline and taxane-based chemotherapy between 2009 and 2015 were included in the study. Data on clinical characteristics and treatment details were collected from the patients’ medical records.
Results: Two hundred sixty-four women were included in the analysis. The median age at presentation was 50 years. Among the 264 women, 40.5% were premenopausal, 1.2% were perimenopausal, and 58.3% were postmenopausal. The number of patients undergoing breast-conserving surgery (BCS) and modified radical mastectomy (MRM) were 35.2% and 64.7%, respectively. Patients with a tumor grade of 1, 2, and 3 were 7.2%, 53.1%, and 39.7%, respectively. Tumors were unifocal in 81.1% and multifocal in 18.2% of patients. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) positivity was detected in 58.3%, 54.2%, and 3.1% of patients, respectively and 38.6% of patients were triple negative. With a median follow-up of 36.2 months, the invasive disease-free survival rate was 90.9% and mean disease-free survival time was 65.4 ± 1.13 months.
Conclusions: The results of this study confirm the clinical utility of anthracycline and taxane-based chemotherapy regimen as the adjuvant chemotherapy treatment of early stage breast cancer.
Keywords: Anthracycline; Taxane; Early breast cancer; Adjuvant chemotherapy
| Introduction | ▴Top |
Breast cancer is the most common malignancy among women with over 1.67 million new cases diagnosed worldwide and 500,000 deaths reported yearly [1-3]. In a 5-year period (2008 - 2012), the incidence rate and mortality of breast cancer have increased by 20% and 14%, respectively. The incidence of breast cancer is higher in developed Western regions compared to developing regions. Incidence rates also vary greatly between regions with rates as high as 89.9 per 100,000 women in Western Europe and as low as 19.3 per 100,000 women in Eastern Africa [4-6]. In India, the age adjusted incidence rate of breast cancer (25.8 per 100,000) is low but the mortality (12.7 per 100,000) is comparable to rates in Western regions [7-9]. Amongst Indian women, breast cancer remains the most common cancer and is the leading cause of cancer-related mortality [10].
The past few decades have witnessed a significant improvement in the survival of women with early breast cancer due to the introduction of adjuvant chemotherapy, endocrine therapy and human epidermal growth factor receptor-2 (HER-2)-directed therapies [11]. The introduction of adjuvant chemotherapy has not only decreased the risks of recurrence in early stage breast cancer, but has also improved the overall survival [12]. The anthracycline and taxane-based chemotherapy regimen is currently being used widely as a standard of care as adjuvant chemotherapeutic treatment of early stage breast cancer [13, 14]. However, knowledge of the use and effectiveness of this treatment regimen in Indian context is limited. In this single-institution study in India, we aimed to understand the clinical characteristics, demographics, and outcomes of the adjuvant anthracycline and taxane-based chemotherapy regimen in routine clinical practice.
| Patients and Methods | ▴Top |
Approval of the Institutional Ethics Committee was obtained for the study. Patients included in the study adhered to the following criteria: 1) Patients with histologically or cytologically confirmed early breast cancer who underwent primary breast surgery; 2) Adjuvant treatment with anthracycline and taxane-based chemotherapy regimen; 3) Admission to the Medanta Cancer Institute between 2009 and 2015. Data on clinical characteristics and treatment details were collected from the patients’ medical records.
The primary efficacy endpoint was invasive disease-free survival (IDFS). The width of the resultant confidence intervals (CIs) for parameters was constructed with a significance level of 0.05 (a 95% CI).
Overall survival (OS) and progression-free survival (PFS) were analyzed with the use of Kaplan-Meier survival analysis, and estimates were provided with 95% CI. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc.).
| Results | ▴Top |
A total of 264 women admitted between August 2009 and July 2015 were included in the study analysis (Table 1). The median age at presentation was 50 years (range, 24 - 76 years). Among the 264 women, 107 (40.5%) were premenopausal, three (1.2%) were perimenopausal, and 154 (58.3%) were postmenopausal. The number of patients undergoing breast-conserving surgery (BCS) and modified radical mastectomy (MRM) were 35.2% and 64.7%, respectively. Estrogen receptor (ER), progesterone receptor (PR) and HER-2 receptor positivity was detected in 58.3%, 54.2% and 3.1% of patients, respectively and 38.6% of patients were triple negative. Although the overall HER-2 receptor positivity at our center is 25.2%, the subset of HER-2 receptor positive patients treated according to the anthracycline and taxane-based chemotherapy regimen represented 3.1% of our focused study population.
 Click to view | Table 1. Summary of Patient Demographic and Clinical Characteristics |
As a part of the study, we tracked the recurrence status of the breast cancer patients (Table 2). With a median follow-up time of 36.2 months (range, 0.2 - 98.7 months), 24 (9.1%) patients experienced recurrence of disease whereas 240 (90.9%) were disease-free. Since, there was an unequal distribution of events and censoring, median IDFS duration could not be estimated (Fig. 1). However, the mean disease-free survival duration was found to be 65.4 ± 1.13 months. The association of each of the individual factor with regards to disease recurrence and disease-free survival are presented in Tables 3 and 4, respectively. While the number of positive nodes was significantly associated with recurrence of disease (P < 0.05), as well as disease-free survival (P < 0.05); there was a decreased association of breast laterality with recurrence of disease (P = 0.06). While some common grade 1/2 toxicities were observed in our study population (Table 5), none of the patient experienced any grade 3/4 toxicities due to the usage of supportive care with granulocyte-colony stimulating factor (G-CSF) and pegylated-G-CSF.
 Click to view | Table 2. Recurrence Status of Patients |
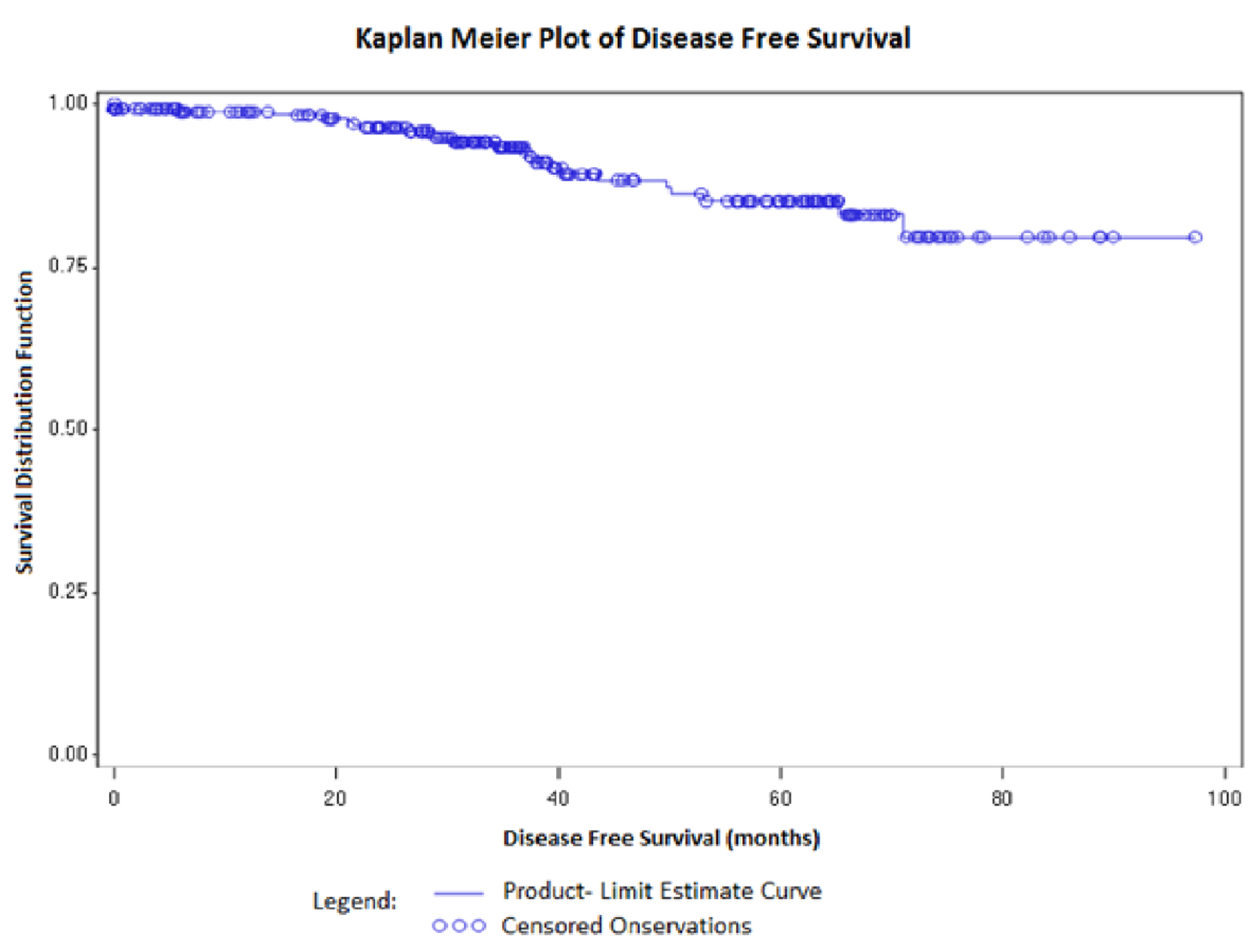 Click for large image | Figure 1. Kaplan-Meier survival analysis for invasive disease-free survival (IDFS). |
 Click to view | Table 3. Cox Regression Analysis for Recurrence of Disease |
 Click to view | Table 4. Cox Regression Analysis for Disease-Free Survival |
 Click to view | Table 5. Common Grade 1/2 Toxicities |
| Discussion | ▴Top |
Chemotherapeutic treatment with anthracyclines (e.g., doxorubicin, epirubicin, liposomal doxorubicin) and taxanes (e.g., docetaxel, paclitaxel, nab-paclitaxel) have shown superiority over the previously used cyclophosphamide, methotrexate and fluorouracil (CMF) combination therapy. Therefore, this treatment strategy is now considered the gold standard for adjuvant chemotherapeutic treatment of early stage breast cancer [13-19]. In an attempt to strengthen the evidence regarding the efficacy of anthracycline and taxane-based treatment, besides the present study, a number of clinical trials have been conducted to evaluate the best agent, dose, and sequence of administration for the treatment of breast cancer [20-24].
In a retrospective study of 1,600 patients, Alvarez et al examined the usual adjuvant sequence of anthracycline followed by taxane and showed a significant higher risk of death compared to the reverse sequence (taxane followed by anthracycline) [25]. Another study reviewed 15 previous studies with a total of nearly 5,000 breast cancer patients treated with anthracyclines and taxanes in an adjuvant or neoadjuvant setting. Their work concluded that taxane followed by anthracycline could be incorporated into routine clinical practice [26].
The adjuvant sequence of anthracycline followed by taxane used in our study is consistent with that followed in previously published reports. The American Society of Clinical Oncology guideline adaptation of the Cancer Care Ontario Clinical Practice guidelines recommends the use of a regimen containing anthracycline and taxane as an optimal adjuvant chemotherapy strategy for patients who are deemed to be at high risk [27]. A 15-year meta-analysis comprising 100,000 women treated across 123 randomized trials demonstrated a reduction in 10-year breast cancer mortality by one-third with anthracycline-based chemotherapy regimens as compared to no chemotherapy [13]. However, anthracycline regimens were associated with increased risk of cardiac mortality, myelodysplastic syndromes and treatment-related leukemia [13, 22]. A series of three randomized adjuvant trials (The ABC Trias-US Oncology Research (USOR) 06-090, National Surgical Adjuvant Breast and Bowel Project (NSABP) B-46-I/USOR 07132 and NSABP B-49 (NRG Oncology)) evaluated the role of anthracyclines in early breast cancer [28]. However, in that series of trials, fewer patients were under the age of 50 years, compared with our study population. This discrepancy could be due to the prevalence of early breast cancer in younger age groups in India compared to Western regions. Additionally, these studies showed a decreased frequency of ≥ 10 positive nodes in the tumors suggesting that more aggressive disease may strike Indian women compared to their Western counterparts.
While the USOR 06-090 study compared six cycles of docetaxel and cyclophosphamide (TC6) with docetaxel, doxorubicin and cyclophosphamide (TAC6), the NSABP B-46-I/USOR 07132 study compared TC6, TAC6 or TC6 plus bevacizumab. On the other hand, the NSABP B-49 study compared TC6 with several standard doxorubicin and cyclophosphamide (AC) with taxanes combination regimens (TaxAC). With a median follow-up of 36.2 months and an IDFS of 90.9%, our study is consistent with findings reported in the NSABP B-49 study.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) conducted a meta-analysis of 10 randomized trials to investigate the long-term outcome for neoadjuvant versus adjuvant chemotherapy in early breast cancer [29]. There was more frequent 15-year local recurrence with neoadjuvant chemotherapy as compared to adjuvant chemotherapy in this study. However, no difference was noted between the two groups for distal tumor recurrence or death. Although, the mean disease-free survival duration of 65.4 ± 1.13 months achieved in our study has not reached the milestone of 10 to 15 years, the results are encouraging with regards to the clinical advantage of adjuvant anthracycline and taxane-based chemotherapy of early stage breast cancer. The results of our study demonstrate the clinical utility of adjuvant anthracycline and taxane-based chemotherapy regimen in early stage breast cancer during the routine clinical practice.
Clinical practice points
Clinical practice points included: 1) Anthracycline and taxane-based chemotherapies are the standard of care for breast cancer treatment; 2) This is the first study examining the effects of anthracycline and taxane-based chemotherapeutic treatment of Indian breast cancer patients; 3) The average age at time of diagnosis for early breast cancer patients was 50 years; 4) With a median follow-up time of 36.2 months, 9.1% of patients had recurrence of disease and the remaining 90.9% were disease-free; 5) The mean disease-free survival time was 65.4 ± 1.13 months; 6) While a portion of the patients experienced grade 1/2 toxicities, none of the population experienced grade 3/4 toxicities due to supportive care with G-CSF based interventions.
Conclusions
Our study is the first Indian cohort focused analysis of the impact of anthracycline and taxane-based adjuvant chemotherapy regimen in the treatment of early stage breast cancer. Further follow-up research will need to be conducted to assess the long-term utility of this treatment regimen. Additionally, analysis of the treatments in the context of a prospective study on the treatment regimen should be conducted. Although our retrospective study has limitations, it confirms the clinical utility of anthracycline and taxane-based chemotherapy regimen in the adjuvant chemotherapy treatment of early stage breast cancer.
Acknowledgments
None to declare.
Financial Disclosure
The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
This study was reviewed by the Institutional Review Board and was exempted from the requirement for informed consent.
Author Contributions
Ashok K. Vaid was responsible for the conception and design of this paper, acquisition of data, analysis and interpretation of data, drafting the article and final approval of the version to be published. Aseem Khurana and Devender Sharma were responsible for the acquisition of data and final approval of the version to be published. Dheeraj Gautam, Jyoti Wadhwa, Rajiv Agarwal, Kanchan Kaur, Jyoti Arora and Kush Gupta were responsible for drafting the article and final approval of the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
doi pubmed - Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374-1403.
doi pubmed - de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607-615.
doi - Launoy G. Epidemiology of cancers in France. Rev Prat. 2010;60(2):178-182.
- Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, Park EC, et al. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009;24(6):995-1003.
doi pubmed - Hery C, Ferlay J, Boniol M, Autier P. Quantification of changes in breast cancer incidence and mortality since 1990 in 35 countries with Caucasian-majority populations. Ann Oncol. 2008;19(6):1187-1194.
doi pubmed - Gupta A, Shridhar K, Dhillon PK. A review of breast cancer awareness among women in India: Cancer literate or awareness deficit? Eur J Cancer. 2015;51(14):2058-2066.
doi pubmed - Sharma K, Costas A, Shulman LN, Meara JG. A systematic review of barriers to breast cancer care in developing countries resulting in delayed patient presentation. J Oncol. 2012;2012:121873.
doi pubmed - Jones CE, Maben J, Jack RH, Davies EA, Forbes LJ, Lucas G, Ream E. A systematic review of barriers to early presentation and diagnosis with breast cancer among black women. BMJ Open. 2014;4(2):e004076.
doi pubmed - Kaarthigeyan K. Cervical cancer in India and HPV vaccination. Indian J Med Paediatr Oncol. 2012;33(1):7-12.
doi pubmed - Cossetti RJ, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. 2015;33(1):65-73.
doi pubmed - Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1992;339(8785):71-85.
doi - Early Breast Cancer Trialists' Collaborative Group, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-444.
doi - Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717.
doi - Wildiers H, Forceville K, Paridaens R, Joensuu H. Taxanes and anthracyclines in early breast cancer: which first? Lancet Oncol. 2010;11(3):219-220.
doi - Puhalla S, Mrozek E, Young D, Ottman S, McVey A, Kendra K, Merriman NJ, et al. Randomized phase II adjuvant trial of dose-dense docetaxel before or after doxorubicin plus cyclophosphamide in axillary node-positive breast cancer. J Clin Oncol. 2008;26(10):1691-1697.
doi pubmed - Piedbois P, Serin D, Priou F, Laplaige P, Greget S, Angellier E, Teissier E, et al. Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol. 2007;18(1):52-57.
doi pubmed - Levine MN, Pritchard KI, Bramwell VH, Shepherd LE, Tu D, Paul N, National Cancer Institute of Canada Clinical Trials Group. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol. 2005;23(22):5166-5170.
doi pubmed - Poole CJ, Earl HM, Hiller L, Dunn JA, Bathers S, Grieve RJ, Spooner DA, et al. Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Engl J Med. 2006;355(18):1851-1862.
doi pubmed - Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663-1671.
doi pubmed - Wood WC, Budman DR, Korzun AH, Cooper MR, Younger J, Hart RD, Moore A, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253-1259.
doi pubmed - Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976-983.
doi pubmed - Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431-1439.
doi pubmed - Swain SM, Jeong JH, Geyer CE, Jr., Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053-2065.
doi pubmed - Alvarez RH, Bianchini G, Hsu L, Cristofanilli M, Esteva FJ, Pusztai L, Buzdar AU, et al. Clinical outcome of two sequences of administering paclitaxel (P) and anthracyclines (A) as primary systemic therapy (PST) and adjuvant chemotherapy (ACT) in breast cancer (BC) patients: a retrospective analysis from the M. D. Anderson Cancer Center (MDACC). Cancer Res. 2010;70(24 Suppl):P5-10-02.
doi - Bines J, Earl H, Buzaid AC, Saad ED. Anthracyclines and taxanes in the neo/adjuvant treatment of breast cancer: does the sequence matter? Ann Oncol. 2014;25(6):1079-1085.
doi pubmed - Denduluri N, Somerfield MR, Eisen A, Holloway JN, Hurria A, King TA, Lyman GH, et al. Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2) -negative and adjuvant targeted therapy for HER2-positive breast cancers: an American society of clinical oncology guideline adaptation of the cancer care ontario clinical practice guideline. J Clin Oncol. 2016;34(20):2416-2427.
doi pubmed - Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE, Jr., Jacobs SA, Robert NJ, et al. Anthracyclines in early breast cancer: the ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017;35(23):2647-2655.
doi pubmed - Early Breast Cancer Trialists' Collaborative Group. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27-39.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


