| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 11, Number 5, October 2020, pages 216-222
Overexpression of Programmed Death-Ligand 1 Receptor mRNA as an Independent Negative Prognostic Factor for Triple Negative Breast Cancer
Ibnu Purwantoa, f, Didik Setyo Heriyantob, Ahmad Ghozalib, Irianiwati Widodob, Iwan Dwiprahastoc, Teguh Aryandonod, Sofia Mubarika Haryanae
aHematology and Medical Oncology Division, Department of Internal Medicine, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University, Yogyakarta, Indonesia
bDepartment of Anatomical Pathology, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr. Sardjito Hospital, Yogyakarta, Indonesia
cDepartment of Clinical Pharmacology, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr. Sardjito Hospital, Yogyakarta, Indonesia
dDepartment of Surgical Oncology, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr. Sardjito Hospital, Yogyakarta, Indonesia
eDepartment of Histology and Cell Biology, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr. Sardjito Hospital, Yogyakarta, Indonesia
fCorresponding Author: Ibnu Purwanto, Hematology and Medical Oncology Division, Department of Internal Medicine, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University, Jalan Farmako, Sekip Utara, Yogyakarta 55281, Indonesia
Manuscript submitted June 8, 2020, accepted January 23, 2020, published online October 15, 2020
Short title: Prognostic Value of PD-L1 in Indonesian TNBC
doi: https://doi.org/10.14740/wjon1302
| Abstract | ▴Top |
Background: Triple negative breast cancer (TNBC) (represents roughly 25% of all breast cancers in Yogyakarta) still has the worst survival compared to other breast cancer subtypes. Results from recent studies have shown that inhibition of programmed death-ligand 1 receptor (PD-L1) in TNBC patients is associated with better prognosis. Currently, data on PD-L1 expression and its prognostic value in Indonesian TNBC patients are still relatively unknown. This study aimed to investigate the expression of PD-L1 in Indonesian TNBC patients as preliminary proof to support PD-L1 inhibitor as a possible treatment option near in the future.
Methods: We retrospectively included stage I-III TNBC patients diagnosed between 2014 and 2017 in Dr. Sardjito Hospital, Yogyakarta, Indonesia. Clinical variables were collected from medical record. Paraffin blocks of biopsy specimen were retrieved to examine mRNA level of PD-L1.
Results: We included 48 subjects with mean age of 51.09 years and mean body mass index (BMI) of 24.58. The 3-year overall survival (OS) was 58.3%. Overexpression of PD-L1 mRNA in TNBC patients is associated with worse prognosis (P < 0.01). There were no statistically significant associations between PD-L1 mRNA expression and any of the clinicopathologic variables examined.
Conclusions: In summary, PD-L1 mRNA overexpression is associated with worse survival in Indonesian TNBC patients, independent of other established risk factors. PD-L1 mRNA is expressed in all of our samples, presenting as a feasible alternative or complementary method in deciding which patient might benefit from receiving PD-L1 inhibitor.
Keywords: PD-L1; Triple negative breast cancer; Prognostic factor; Indonesia
| Introduction | ▴Top |
Triple negative breast cancer (TNBC) is immunohistochemically defined as the lack of estrogen receptor (ER) and progesterone receptor (PR) expression accompanied with the lack of human epidermal receptor 2 (HER2) overexpression [1]. Collectively TNBC has the worst survival compared to other breast cancer subtypes [2-7]. TNBC accounts for 10-20% of all breast cancers, which roughly translates into 200,000 new cases every year worldwide [8, 9]. Currently, there are no data on the prevalence of TNBC in Indonesia. The estimated incidence of breast cancer (BC) in Indonesia is 18.6/100,000 females annually [10]. A study reported that TNBC accounts for 25% of all breast cancer cases at Dr. Sardjito Hospital, Yogyakarta, Indonesia [11].
The rise of immunotherapy has opened new opportunities in TNBC treatment, especially with programmed death-ligand 1 receptor (PD-L1) inhibition. PD-L1, an immune checkpoint receptor, is expressed higher in patients with TNBC when compared to non-TNBC patients and is associated with worse prognosis. Results from recent studies have shown that inhibition of PD-L1 in TNBC patients is associated with improved prognosis, both as monotherapy in metastatic setting and in combination with systemic chemotherapy in neoadjuvant setting [12-15]. However, NeoTRIP trial showed that the addition of atezolizumab to carboplatin and nab-paclitaxel in early high-risk and locally advanced TNBC did not improve pathologic complete response (pCR) when compared to chemotherapy alone, suggesting the benefit of PD-L1 inhibitors might be affected by several factors, including PD-L1 expression, type of chemotherapy back-bone and disease stage [16].
Currently, PD-L1 expression and its prognostic value in Indonesian TNBC patients is still relatively unknown. This study aimed to investigate the expression of PD-L1 in Indonesian TNBC patients as preliminary proof to support PD-L1 inhibitor as a possible treatment option near in the future.
| Materials and Methods | ▴Top |
This study was a retrospective cohort study conducted at Dr. Sardjito Hospital, Yogyakarta, Indonesia. TNBC patients diagnosed in 2014 - 2017 were selected using a consecutive sampling method. Among 106 diagnosed patients, only 48 patients met the inclusion criteria with retrievable paraffin block for analysis. The inclusion criteria were patients with hormone receptor negative and HER2 negative; no history of inflammatory and immune disease; no history of diabetes mellitus, hypertension, cardiac, renal and hematological diseases. Patients with distant metastasis, inflammatory breast tumor, bilateral breast cancer and infectious diseases were excluded from this study. Tumor samples were obtained from formalin-fixed paraffin-embedded (FFPE) tissue stored at the Department of Anatomical Pathology, Dr. Sardjito General Hospital, Yogyakarta, Indonesia; Waskhita Laboratory, Yogyakarta, Indonesia; Panti Rapih General Hospital, Yogyakarta, Indonesia; and CITO Laboratory, Yogyakarta, Indonesia. Expression of PD-L1 mRNA was determined by using quantitative real-time polymerase chain reaction (qRT-PCR). We chose qRT-PCR over immunohistochemistry (IHC) since automated IHC examination, especially for PD-L1, was uncommon in Indonesia and we avoided to perform IHC manually due to its subjective nature and lack of standardization. For preparation of RNA from FFPE samples, the RNeasy FFPE kit (QIAGEN GmbH, Hilden, Germany) was used according to manufacturer’s recommendations. Three sections of 10 µm FFPE thickness were used per preparation. For quantitative PCR (qPCR), RNA/sample was amplified using the NEXpro™ qRT-PCR Master (SYBR) (Cat. No. NexQ-7000) in a Step One Real Time PCR System (BioneerExicyclerTM 96 Real Time Quantitative Thermal Block). PD-L1 forward primer, 5’-TATGGTGGTGCCGACTACAA-3’, and PD-L1 reverse primer, 5’-TGGCTCCCAGAATTACCAAG-3’. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been used for normalization of gene expression data. The cycling conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 20 s, annealing at 60 °C for 40 s and extension at 72 °C for 60 s. Clinical data and survival status were retrieved from medical records. Cutoff value for PD-L1 mRNA was determined by using receiver operating curve (ROC) to calculate area under the curve (AUC) and Youden index. Chi-square analysis was used to compare the expression of PD-L1 mRNA within each category of clinical characteristics. Survival analysis was performed using Kaplan-Meier and Cox proportional hazard. All procedures of the present study were conducted in compliance with the Helsinki Declaration for research on human beings. This study has been approved by the IRB Ethics Committee Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr. Sardjito Hospital, Yogyakarta, Indonesia.
| Results | ▴Top |
From 106 diagnosed patients, 48 subjects were eligible for analysis. The mean age of the subjects was 51.09 years, while the median age was 50.24 years. Patients were divided into two groups according to its median value, 21 (43.8%) subjects were below 50 years old and 27 (56.3%) subjects were more than or equal to 50 years old. Of these 48 patients, the mean body mass index (BMI) was 24.58 and the median was 24.00. Fifteen (31.3%) subjects had BMI < 23 and 33 (68.8%) subjects had BMI ≥ 23. Of the patients, 45.8% were classified into early breast cancer (stage I and II) and the remaining 54.2% were classified into locally advanced breast cancer (stage IIIA-IIIC). From histological examination, 62.5% was classified into grade 3, while 37.5% was classified into grade 1 and 2. Twenty-three patients (47.9%) received platinum-based chemotherapy, while the other 25 patients (52.10%) received anthracycline-based regimen. Twenty-seven patients (56.3%) were given chemotherapy before 60 days, while 21 patients (43.8%) received chemotherapy more than 60 days after diagnosis (Table 1).
 Click to view | Table 1. The Clinicopathological Features of 48 TNBC Patients |
Out of 48 patients included in this study, 28 patients (58.3%) were alive at 3-year follow-up, while 20 patients (41.7%) passed away (Fig. 1). Kaplan-Meier survival curve of 48 TNBC patients is shown in Figure 2, although the median of the survival was not yet achieved. All of the subjects expressed PD-L1 mRNA. Cutoff value of 46.875 was used to classify PD-L1 mRNA expression (Table 2, Fig. 3), with 38 patients (79%) having PD-L1 mRNA underexpression, while the remaining 10 patients (21%) had PD-L1 mRNA overexpression. Worse prognosis was observed in patients with PD-L1 mRNA overexpression (P < 0.01) (Fig. 4). We further stratified PD-L1 mRNA expression according to clinical features such as age, BMI, histological grade, stage, chemotherapy regimen and time from diagnosis to chemotherapy, although significant association was not observed in any of the included variables (Table 3) (Supplementary Material 1, www.wjon.org).
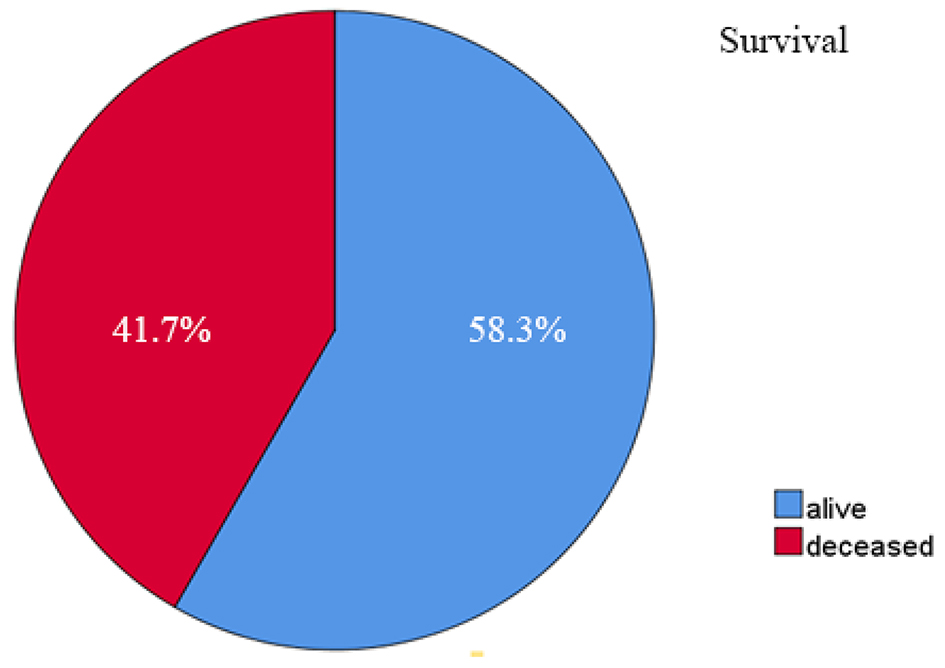 Click for large image | Figure 1. Three-year survival rate of 48 TNBC patients. TNBC: triple negative breast cancer. |
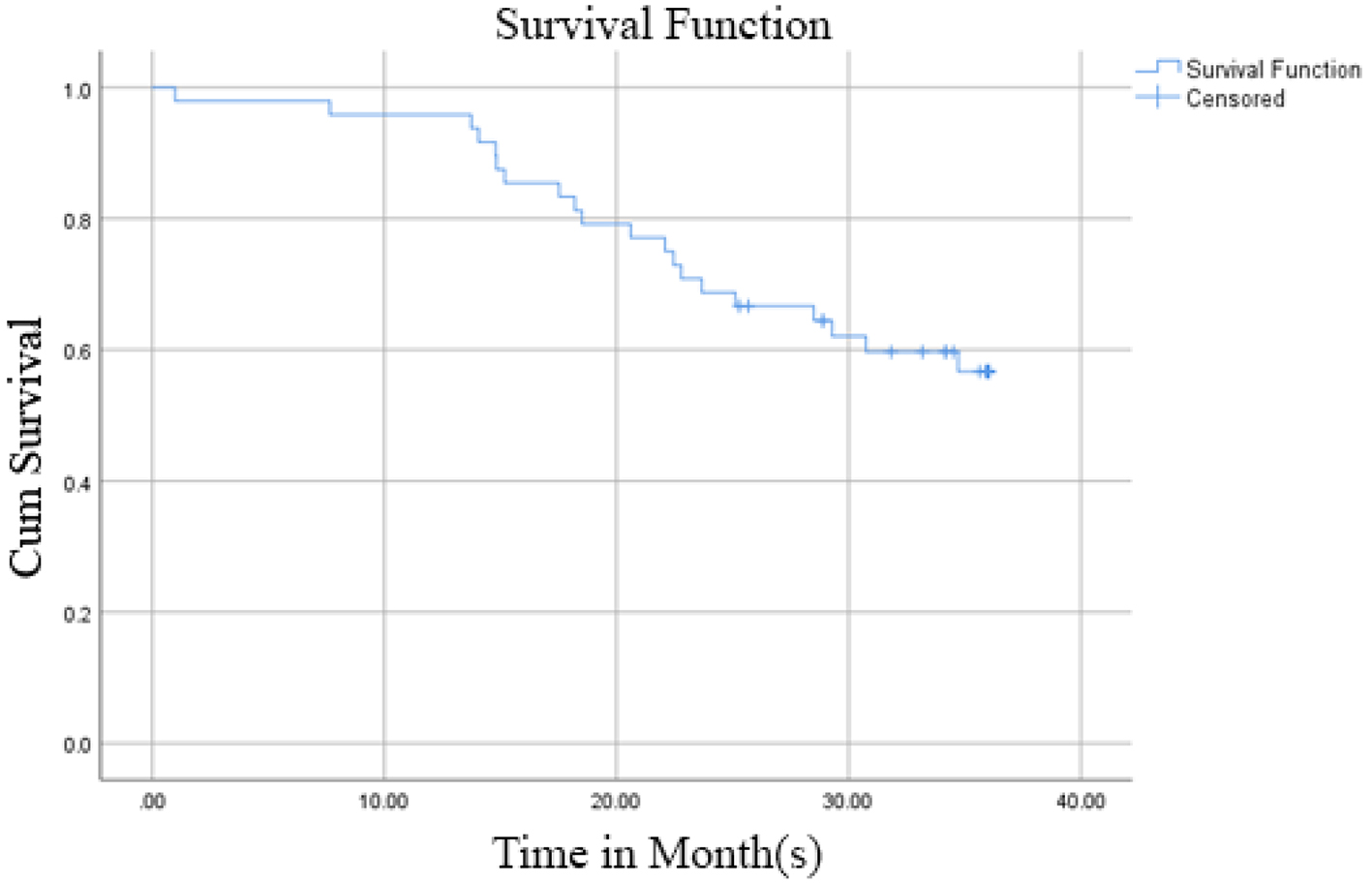 Click for large image | Figure 2. Kaplan-Meier survival curve of 48 TNBC patients. TNBC: triple negative breast cancer. |
 Click to view | Table 2. Determination of Optimal PD-L1 Cutoff Value |
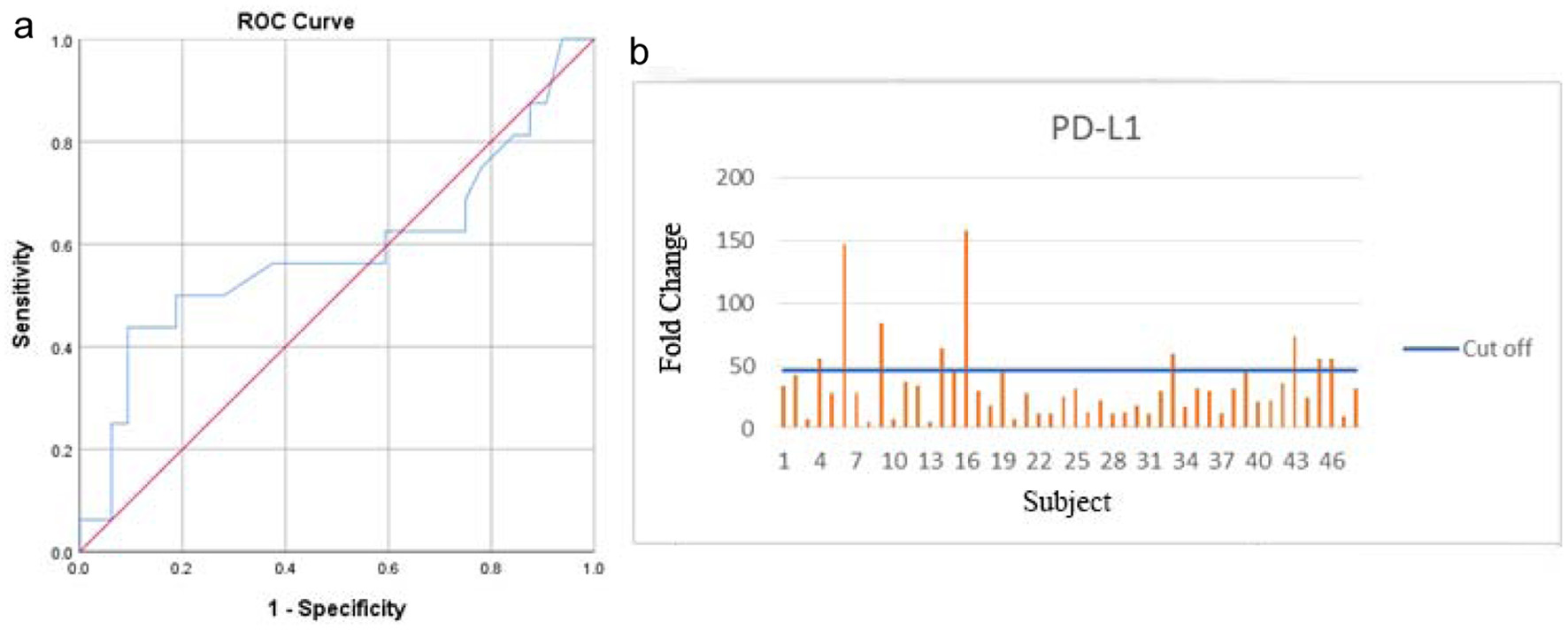 Click for large image | Figure 3. Area under the ROC curve (a) and PD-L1 qRT-PCR result (b). ROC: receiver operating curve; PD-L1: programmed death-ligand 1 receptor; qRT-PCR: quantitative real-time polymerase chain reaction. |
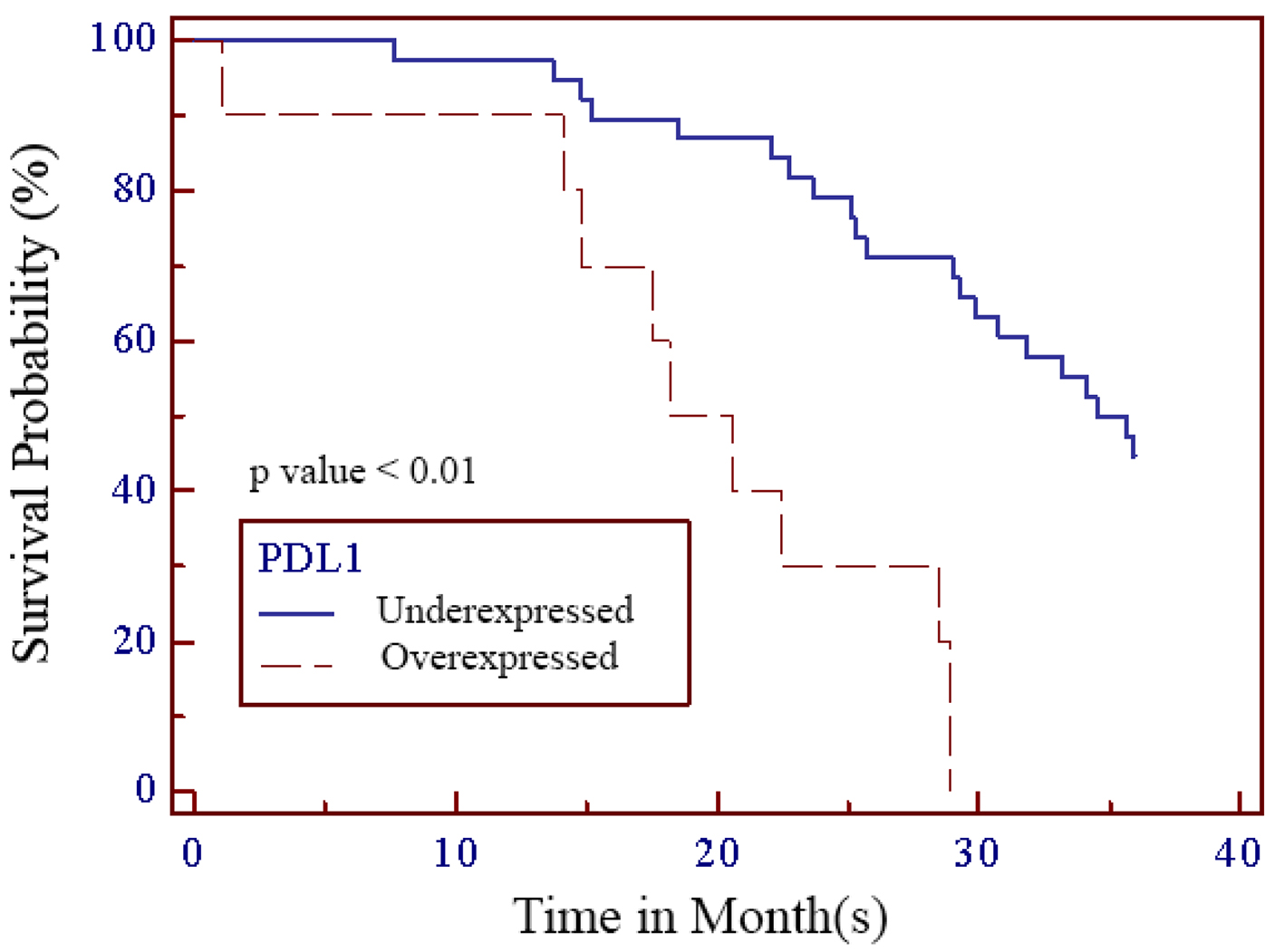 Click for large image | Figure 4. Kaplan-Meier survival curve of PD-L1 in TNBC patients. PD-L1: programmed death-ligand 1 receptor; TNBC: triple negative breast cancer. |
 Click to view | Table 3. Association Between PD-L1 Expression and Clinical Features in TNBC Patients |
| Discussion | ▴Top |
TNBC subtype has an epidemiological, histopathological and clinical presentation different from other breast cancer subtypes. The characteristics of TNBC patients in Yogyakarta (Indonesia) described in this study showed similar feature pattern with TNBC patients in Bandung (Indonesia), including age at diagnosis, as well as clinical and histological characteristics [17]. Although it is important to note that Indonesia not only has numerous population (fourth most numerous in the world) but also heterogenous (comprised of more than 730 ethnic groups), which might have influence on prognosis and response towards chemotherapy [18]. We observe that Indonesian TNBC patients tend to be older and have better prognosis compared to African American patients, suggesting race and genetic profile are important risk factors as well as prognostic factors in TNBC [1]. The 3-year OS in our study was 58.3% which is lower than the 85% rate reported by Srimuninnimit et al [19].
Programmed death-1 receptor (PD-1) is an immune checkpoint receptor and when bound to its PD-L1 ligand, results in immunoinhibitory response which contributes to cancer cell survival and progression [20-23]. In PD-L1 expressing cancers, PD-L1 inhibition results in cancer cell death. On protein level, PD-L1 expression in TNBC tends to be higher when compared to non-TNBC with estimated frequency of 20-58% in all TNBC patients [24-28]. This trend is maintained on mRNA level. Previous studies reported higher PD-L1 mRNA level in TNBC when compared to non-TNBC, although the actual frequency of PD-L1 expression on mRNA level is consistently higher than on protein level [23, 24, 29]. In our study, all of our patients expressed PD-L1 mRNA with 38 patients (79%) having PD-L1 mRNA underexpression, while the remaining 10 patients (21%) had PD-L1 mRNA overexpression. Overexpression of PD-L1 mRNA in our cohort is associated with worse survival (P < 0.01). Similar to our study, Ren et al reported significant association between higher expression of PD-L1 mRNA with worse prognosis. In this study, PD-L1 mRNA positivity using in situ hybridization was 74.4% but PD-L1 protein positivity according to IHC was only 6.7% [23].
Although current evidence on the prognostic value of PD-L1 of TNBC is still conflicting, it is generally accepted that inhibition of this receptor results in better treatment response [12-14, 30]. The discrepancy in the prevalence and clinical implications of PD-L1 expression could be attributed to the different methods to check the expression, variability in the different tumor types, and an inadequately defined evaluation score. The currently used IHC method to determine PD-L1 positivity is not yet perfect. As described by previous studies, PD-L1 inhibitor still results in some therapeutic benefit in patients with < 1% PD-L1 expression, albeit less pronounced than in > 1% expression [31, 32]. Examination method using mRNA might be a feasible alternative or complementary method in determining PD-L1 positivity and deciding which patient might benefit from receiving PD-L1 inhibitor, as mRNA of PD-L1 can be detected even in IHC negative tumor cells [24, 29].
Inconsistent results across similar studies also suggest possibilities that timing and tumor microenvironment might have influence. In our study PD-L1 mRNA overexpression is not associated with other established prognostic factors such as age, BMI, tumor/node/metastasis (TNM) and cancer stage.
This study has several limitations. Our study is a retrospective cohort study with limited sample size which requires follow-up study to validate its result. The small sample size that we have was in part contributed by the widespread storage of tissue sample in multiple labs which made tissue retrieval challenging. The amount of tissue stored from each patient was limited thus not enough to perform in-depth analysis. Comparative analysis towards IHC, the traditionally used method to determine PD-L1 positivity, was not performed in this study. We were able to collect slides only from patients diagnosed in 2014 - 2017, which thus only allows for 3-year survival analysis.
Conclusions
In summary, PD-L1 mRNA overexpression is associated with worse survival in Indonesian TNBC patients, independent of other established risk factors. PD-L1 mRNA is expressed in all of our samples, presenting as a feasible alternative or complementary method in determining which patient might benefit from receiving PD-L1 inhibitor. However, method standardization for PD-L1 mRNA testing is needed prior to routine implementation.
| Supplementary Material | ▴Top |
Suppl 1. Research data.
Acknowledgments
The authors express the upmost gratitude towards Professor Iwan Dwiprahasto who recently passed away due to COVID-19, for his massive contribution not only towards this research but also towards Indonesian health service and education. We also thank Althaf Setyawan for his technical assistance in the statistical analysis. Last but not the least, we extend our gratitude towards all of the supporting assistants who have made this research possible.
Financial Disclosure
The authors received no financial support for the authorship and/or publication of this research.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Written informed consent was obtained from the patients involved, including permission to use clinical data and reexamination of tissue specimen.
Author Contributions
IP was involved in the work conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing original draft and its subsequent revisions. DSH, AG and IW were involved in data curation, formal analysis, investigation and validation. ID was involved in conceptualization, methodology and supervision. TA and SM were involved in conceptualization, investigation, methodology, supervision and reviewing original draft.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7(12):683-692.
doi pubmed - Kaplan HG, Malmgren JA. Impact of triple negative phenotype on breast cancer prognosis. Breast J. 2008;14(5):456-463.
doi pubmed - Lee JA, Kim KI, Bae JW, Jung YH, An H, Lee ES, Korean Breast Cancer S. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat. 2010;123(1):177-187.
doi pubmed - Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429-4434.
doi pubmed - Nishimura R, Arima N. Is triple negative a prognostic factor in breast cancer? Breast Cancer. 2008;15(4):303-308.
doi pubmed - Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329-2334.
doi pubmed - Yuan N, Meng M, Liu C, Feng L, Hou L, Ning Q, Xin G, et al. Clinical characteristics and prognostic analysis of triple-negative breast cancer patients. Mol Clin Oncol. 2014;2(2):245-251.
doi pubmed - Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876-884.
doi pubmed - Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721-1728.
doi pubmed - Wahidin M, Noviani R, Hermawan S, Andriani V, Ardian A, Djarir H. Population-based cancer registration in Indonesia. Asian Pac J Cancer Prev. 2012;13(4):1709-1710.
doi pubmed - Widodo I, Dwianingsih EK, Triningsih E, Utoro T, Soeripto. Clinicopathological features of indonesian breast cancers with different molecular subtypes. Asian Pac J Cancer Prev. 2014;15(15):6109-6113.
doi pubmed - Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460-2467.
doi pubmed - Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, Grischke EM, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279-1288.
doi pubmed - Nanda R, Liu MC, Yau C, Asare S, Hylton N, Veer LV, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J Clin Oncol. 2017;35(15_suppl):506-506.
doi - Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821.
doi pubmed - Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, Thill M, et al. Abstract GS3-04: Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Cancer Res 2020;80(4 Suppl):GS3-04.
doi - Akwarini F, Fadjari TH, Hernowo BS. Original article triple negative breast cancer characteristics based on basal-like and non-basal-like subtypes. International Journal of Integrated Health Sciences. 2019;7(1):26-33.
- Purwanto I, Dwiprahasto I, Aryandono T, Mubarika S. Treatment options for Indonesian triple negative breast cancer patients: a literature review of current state and potentials for future improvement. J Med Sci. 2020;52(1):81-101.
doi - Srimuninnimit V, Pornpraserthsuk P, Chaiwerawattana A, Kongdan Y, Namkanisorn T, Somwangprasert A, Jatuparisuthi C, et al. Real-life clinical pattern, management, and survival in Thai patients with early-stage or metastatic triple-negative breast cancer. PLoS One. 2018;13(12):e0209040.
doi pubmed - Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887-3895.
doi pubmed - Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153-167.
doi pubmed - Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561-584.
doi pubmed - Ren X, Wu H, Lu J, Zhang Y, Luo Y, Xu Q, Shen S, et al. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol Ther. 2018;19(5):373-380.
doi pubmed - Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361-370.
doi pubmed - Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190-198.
doi pubmed - Schmid P, Park YH, Munoz-Couselo E, Kim S-B, Sohn J, Im S-A, et al. Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): Preliminary results from KEYNOTE-173. J Clin Oncol. 2017;35(15_suppl):556-556.
doi - Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, Overberg P, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965-2970.
doi pubmed - Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70.
doi pubmed - Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, Holliday A, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69(1):25-34.
doi pubmed - Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, Zhang Q. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8(19):31347-31354.
doi pubmed - Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, Kuter I, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74-82.
doi pubmed - Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, Cescon DW, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397-404.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


