| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 2, April 2022, pages 53-58
Impact of the Discordance Between Scales of Memorial Sloan-Kettering Cancer Center and International Metastatic Renal Cell Carcinoma Database Consortium in Patients’ Prognosis With Metastatic Renal Cancer
Hiram Josue Grimaldo-Roquea, Erika Adriana Martinez-Castanedaa, Mariana G. Morales-Garciaa, Jorge Luis Leal-Hidalgoa, Valeria M. Torres-Guillenc, Rita Dorantes-Herediab, Daniel Motola-Kubaa, Jose Manuel Ruiz-Moralesa, d
aMedical Oncology Research Unit, Medica Sur Hospital and Clinical Foundation, Mexico City, Mexico
bAnatomic Pathology Research Unit, Medica Sur Hospital and Clinical Foundation, Mexico City, Mexico
cUniversidad de las Americas Puebla, San Andres Cholula, Puebla, Mexico
dCorresponding Author: Jose Manuel Ruiz-Morales, Medical Oncology Research Unit, Medica Sur Hospital and Clinical Foundation, Mexico City, Mexico
Manuscript submitted September 22, 2021, accepted April 6, 2022, published online April 23, 2022
Short title: Conflict in Patients’ Prognosis With mRCC
doi: https://doi.org/10.14740/wjon1400
| Abstract | ▴Top |
Background: In Mexico, about 30% of renal cancer patients are diagnosed in a metastatic state. Despite the recent advances in the treatment of cancer, metastatic renal cancer is still an incurable illness. Thus, identifying prognostic factors helps improve prognosis accuracy and survival prediction for patients.
Methods: In this study, we retrospectively analyzed 26 patients with histological diagnosis of renal cell carcinoma, including clear cell and other subtypes in stage IV (metastatic), recurrent or unresectable disease. We performed a multivariate analysis of overall survival regarding the congruity between prognostic scales.
Results: Our results showed a significant difference in favor of patients with congruity between scales for progression-free survival (18.9 vs. 3.1 months; P = 0.048) and a tendency towards better overall survival in patients with the congruity of both scales compared to the discordant patients (112 vs. 32 months; P = 0.99).
Conclusion: This study highlights the discordance between Memorial Sloan-Kettering Cancer Center and International Metastatic Renal Cell Carcinoma Database Consortium scales, which was associated with worse prognosis with a significant difference in progression-free survival but not in overall survival.
Keywords: Prognosis; Renal cell cancer; Targeted therapy
| Introduction | ▴Top |
Renal cancer represents 2% of cancer diagnoses worldwide [1]. According to GLOBOCAN 2018, renal cancer occupies the 16th place in incidence and accounts for 1.8% of global cancer deaths [2]. It is the 15th most common cancer in Mexico and the 12th leading cause of mortality. It is assumed that the incidence of this cancer in Mexico is underestimated [3].
In developed countries, most of renal tumors are diagnosed incidentally. In Mexico, 30% of the patients are diagnosed in the metastatic state, compared with 16% in the USA. Moreover, although the overall incidence of renal cancer in Mexico is similar to that of the rest of the world, the country has a higher mortality rate (1.8% vs. 3.3%). Also, approximately 20-35% of patients with initially localized cancer are more likely to have metastatic recurrence and 5% would have local recurrence [4].
Recently, Reynoso-Noveron and Mohar [5] explored the extent of cancer in Mexico using official national data for the period 2005 - 2012. They identified 74,402 cases, of which renal cancer accounted for 1.7%. They did not, however, provide major information regarding advanced renal carcinoma nor its treatment.
At present, many therapeutic options are used for the metastatic stage, such as tyrosine kinase inhibitors (TKIs), antiangiogenic therapy and mammalian target of rapamycin (mTOR) inhibitors. Recently, immunotherapy has joined this list as a critical pillar of cancer treatment with excellent outcomes. Despite these recent advances in the treatment of cancer, metastatic renal cancer remains an incurable illness. Therefore, identifying prognostic factors would enhance prognostic accuracy for metastatic renal cancer and prediction of life expectancy for patients with this cancer [6].
The treatment selection in patients with advanced cancer must consider prognostic factors. Several prognostic models have been designed to identify high-risk patients with renal cancer. One of the most widely used models is the Memorial Sloan Kettering Cancer Center (MSKCC), developed by Motzer [6]. MSKCC identifies three risk categories: favorable risk (without factors of risk, median overall survival (mOS) of 20 months), intermediate (one to two factors, mOS of 10 months) and poor risk (three or more factors, mOS of 4 months). Factors associated with less survival include anemia, hypercalcemia, Karnofsky performance score < 80%, time of diagnosis to treatment < 1 year, lactate dehydrogenase > 1.5 times normal upper limit and previous nephrectomy. This risk model was utilized to analyze patients in clinical studies with alpha-interferon [7].
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) proposed new criteria to evaluate the prognosis of patients with renal cancer [8]. The IMDC model considers the following prognostic factors (including four of them proposed by the scale of MSKCC): Karnofsky scale < 80%; time from diagnosis to systemic treatment < 1 year, low hemoglobin, high calcium, neutrophilia and thrombocytosis. The model was validated in more than 600 patients who were treated first-line with anti-vascular endothelial growth factor (VEGF) therapy. Similar to the MSKCC model, IMDC identifies three risk categories: favorable (without factors of risk, mOS of 43 months), intermediate (one or two risk factors, mOS of 22.5 months) and poor risk (three or more factors, mOS of 7.8 months) [9].
Nowadays, the full impact of conflict between both risk scales in patients’ prognosis with renal cell carcinoma remains unknown. Moreover, despite the advances in the systemic treatment of advanced stage of renal cell carcinoma had improved the prognosis of the disease, there is a lack of information about the experience in the use of guided and immunotherapy for renal cancer in Mexico.
| Materials and Methods | ▴Top |
We conducted an observational, descriptive, transverse, retrospective, non-randomized study in patients treated with systemic therapy in our Cancer Center, located in Mexico City. This study was approved by our scientific and bioethical committee (CONBIOETICA). Given the retrospective nature of this analysis, no intervention was made in the study population. Kaplan-Meier analysis was used to analyze the OS. All statistical analyses were performed using IBM SPSS Statistics 25.
Patient selection and demographics
Patients ≥ 18 years were eligible to be included in this study if they had a histological diagnosis of renal cell carcinoma, including clear cell and other subtypes, stage IV (metastatic), recurrent or unresectable disease. Exclusion criteria involved other histological diagnoses such as collecting tubules or urothelial carcinoma, metastatic renal cancer of a primary one in another place and local or locally advanced renal cell carcinoma without evidence of metastases disease.
Outcome measures
The primary purpose of this study was to evaluate the impact of the incongruence between the MSKCC and IMDC risk models on progression-free survival (PFS) and OS. We performed a multivariate analysis of OS with regard to the congruity between the two prognostic scales. The OS is defined as the time from the diagnosis of the illness up to the date of death or last contact with the patient. Secondary goals were to determine the incidence of metastatic renal cancer in our medical institution and to evaluate the use of systemic treatment in first- and second-line context.
| Results | ▴Top |
Patient characteristics
We reviewed kidney surgical case reports performed between January 2012 and June 2019 in the pathology laboratory. We identified 248 samples of nephrectomies and biopsies with a definitive diagnosis of renal cell carcinoma, including the histological subtypes. A total of 44 samples were identified as distant recurrence or metastatic stage. Twenty-six patients accomplished inclusion criteria and were included in the final analysis. Of these patients, 30.8% were females and 69.2% males. The median age was 65 years. The most common histology was clear cell carcinoma (88%). About 53% of the eligible patients were diagnosed in an advanced stage, and 24 of 26 (92%) patients had a nephrectomy performed. Fifty-three percent received second-line treatment and 30% received a third-line treatment. TKIs, mainly pazopanib, were more often used in first- and second-line therapy (Table 1).
 Click to view | Table 1. General Characteristics of the Study Population |
Primary outcome evaluation
The mOS of the studied population was 91.1 months. A disagreement between MSKCC and IMDC scales was observed in 10 of 26 patients (38%). The most common reclassification of risk was from intermediate to poor risk in five patients (19%) (Table 2). We found a statistically significant difference in favor of patients with congruity between scales for PFS (18.9 vs. 3.1 months; P = 0.048) (Fig. 1) and a tendency towards better OS in patients with the congruity of both scales opposite to the discordant patients (112 vs. 32 months; P = 0.99) (Table 3 and Fig. 2).
 Click to view | Table 2. Risk Group According to MSKCC and IMDC Scales |
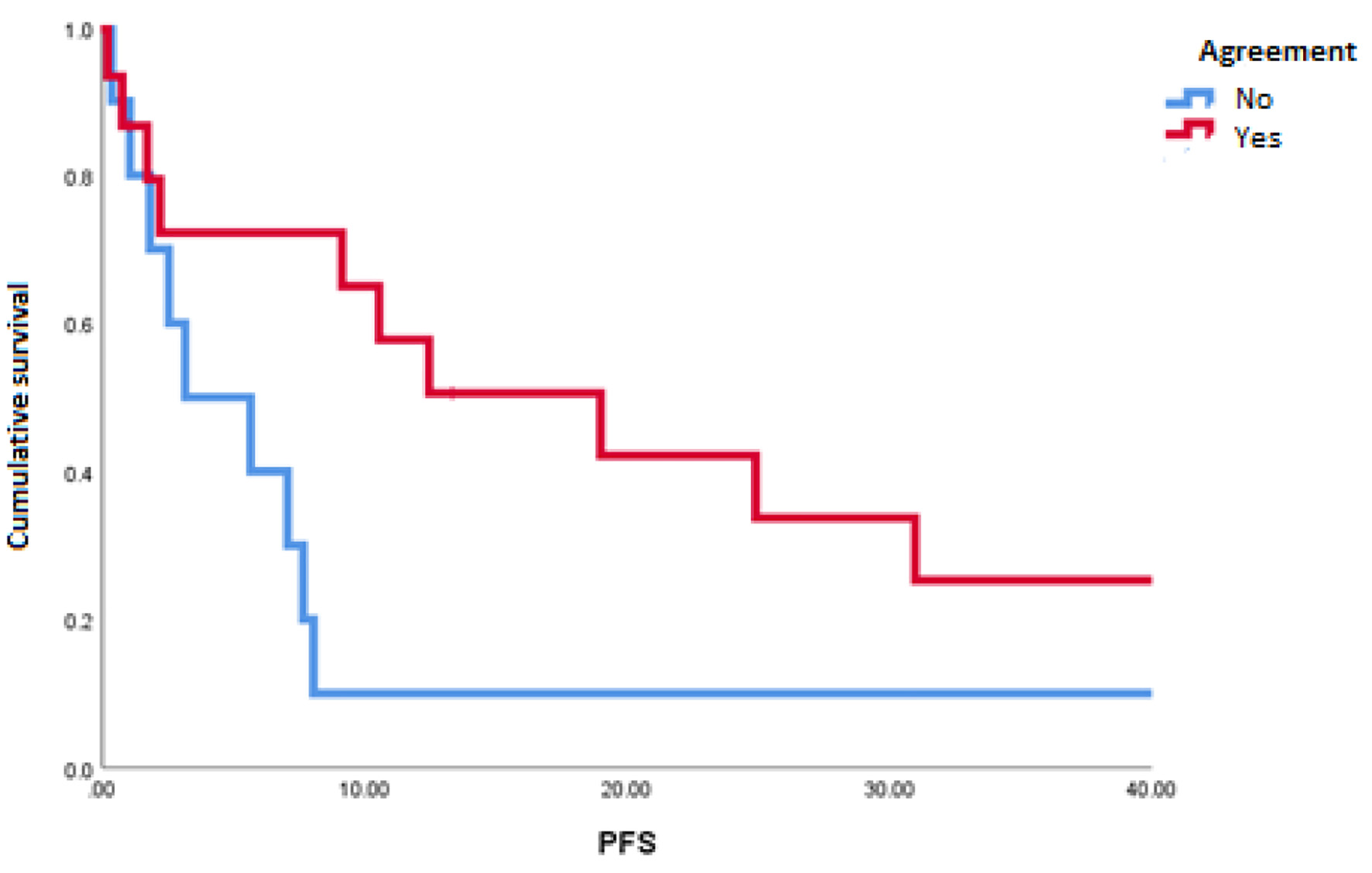 Click for large image | Figure 1. Progression-free survival in patients with agreement and discordance between scales of Memorial Sloan-Kettering Cancer Center and International Metastatic Renal Cell Carcinoma Database Consortium. |
 Click to view | Table 3. Agreement and Discordance Between Scales of MSKCC and IMDC |
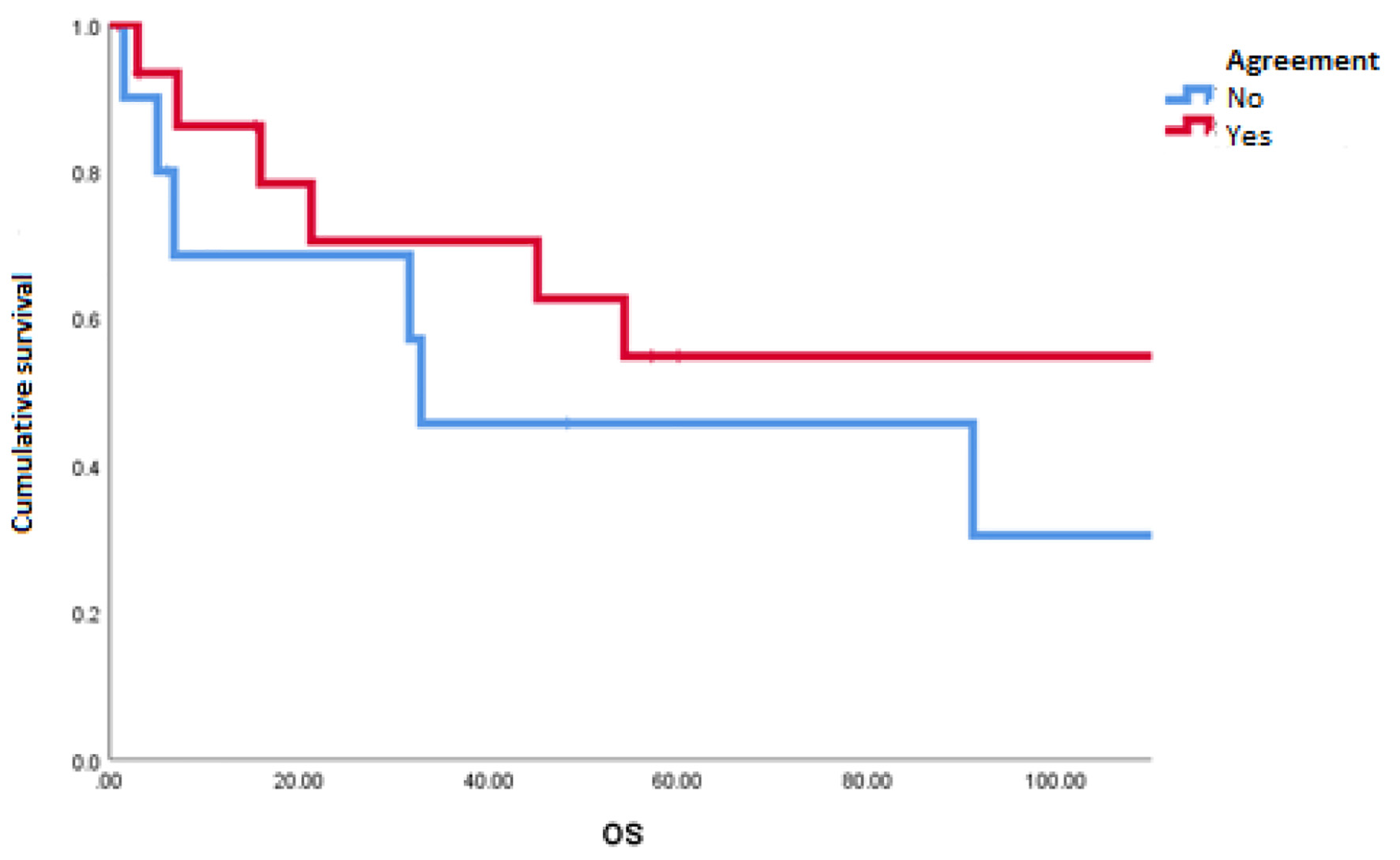 Click for large image | Figure 2. Overall survival in patients with agreement and discordance between scales of Memorial Sloan-Kettering Cancer Center and International Metastatic Renal Cell Carcinoma Database Consortium. |
| Discussion | ▴Top |
Up to 20-40% of patients who were candidates for curative intent surgery for localized renal cell carcinoma will experience disease relapse [10]. The development and correct application of prognostic scales are critical to the treatment of patients in metastatic context (mainly in clinical practice, both also for inclusion in clinical trials). The traditional models include the MSKCC model and the IMDC prognostic model, although other models have been developed, including the Cleveland Clinic Foundation and French Model; all of them include laboratory and biochemical parameters and a measure of performance statues [6].
In 1999, Motzer et al evaluated in a retrospective manner the relationship between the pretreatment clinical features and survival among a cohort of 670 patients with advanced renal cell carcinoma; these patients were treated on 24 MSKCC clinical trials between 1975 and 1996, the results were examined, and a model was developed to stratify patients according to risk of survival. This study resulted in a model based on five pretreatment clinical features that predicted survival for patients with advanced renal cell carcinoma: low Karnofsky (< 80%), high lactate dehydrogenase (1.5 times upper limit of normal (ULN)), low serum hemoglobin (< lower limit of normal), high correct serum calcium (10 mg/dL) and absence of prior nephrectomy. These factors were used to stratify patients into three groups and establish a 3-year survival for each one: favorable risk 31% (no risk factors), intermediate risk 7% (one to two risk factors), and poor risk 0% (three or more risk factors). The MSKCC model was after applied in external data from a trial by Eastern Cooperative Oncology Group; in this trial, the median survival times of favorable, intermediate and poor risk patients were 29, 14 and 4 months, respectively [11, 12].
In the era of VEGF-targeted therapies, the IMDC prognostic model was created. In this model, Heng et al evaluated the characteristics and outcomes of 645 patients with anti-VEGF therapy-naive metastatic renal cell carcinoma from US and Canadian centers, after a Cox proportional hazards regression, followed by bootstrap validation, was used to identify prognostic factors for 2-year OS. Four of the five adverse prognostic factors of MSKCC prognostic model were independent predictors of short survival: hemoglobin less than the lower limit of normal, corrected calcium greater than the ULN, Karnofsky performance status less than 80% and time form diagnosis to treatment of less than 1 year. In addition, neutrophils greater than the ULN and platelets greater than the ULS were independent adverse prognostic factors. Patients were classified into three risk categories: favorable-risk group, with mOS not reached and 2-year OS of 75% (no prognostic factors); the intermediate-risk group, with mOS of 27 months and 2-year OS of 53% (one to two factors); and the poor-risk group (three to six factors), with mOS of 8.8 months and 2-year OS of 7% [13]. The external validation was realized in a cohort of 1,028 patients, of whom 849 had complete data to assess this model and the risk factors were independent predictors of poor OS in the external validation set. The concordance index of the MSKCC model was 0.657 [14], and after this evaluation, the IMDC model could be applied to stratify patients by risk in clinical trials and determining their prognosis.
Although MSKCC classification was developed using data from patients treated with cytokines, there are validation studies of its profit in patients treated with VEGF-targeted therapies (e.g., sunitinib) [15], and assuming that both, MSKCC and IMDC, were established using similar methodology and there is evidence that they are comparable, current guidelines advocate the use of a model but do not recommend one in particular [16].
Little research has been realized about the agreement or discordance in risk-group classification between the MSKCC and IMDC classification and it should be an important research issue, because of the survival in each risk-group is different and the management recommendations, too. Recently, Hatakeyama et al [17] conducted a retrospective study on 176 patients with metastatic renal cell carcinoma that were treated with systemic therapy with TKIs and had a disagreement between MSKCC and IMDC risk scales. The percentage of patients with agreement, upgrade and downgrade was 77%, 22% and 1.1%, respectively. The reclassification from the MSKCC-intermediate to the IMDC-poor risk group was the most frequent. This finding represented a significantly poorer prognosis in patients with disagreement than in those with agreement [17]. In our study, 38% patients had a conflict between both risk models, with intermediate to poor risk being the most common reclassification (19%); a worse prognosis was observed in patients with the conflict between both scales. In another study by Okita et al [18], the IMDC scale re-classified more patients to the poor-risk group (19% of patients). Likewise, significant differences were observed in PFS (17 vs. 5.7 months; hazard ratio (HR) 1.86, P = 0.025), OS (35 vs. 18 months; HR 1.75; P = 0.028) and cancer-specific survival (35 vs. 18 months; HR 1.71, P = 0.040) between the concordant and discordant cohorts [18].
We found that approximately half of the patients were diagnosed at an advanced stage, 92% of patients had a nephrectomy and 54% were performed in the metastatic state. All of our patients received first-line treatment. The discordance between both scales of MSKCC and IMDC was associated with a worse prognosis (112 vs. 32 months; P = 0.99, and the re-classification from intermediate- to poor-risk the most common change in a 19%). A significant difference in PFS but not in OS was observed. This is probably due to the limited number of analyzed patients.
Conclusion
To the best of our knowledge, this study is the first to analyze the discordance between MSKCC and IMDC risk scales for metastatic renal cell carcinoma in a western population (Mexico). Our study confirms that discordance between prognosis risk scores confers a worse prognosis in PFS. Therefore, this information needs to be evaluated with prospective trials and a larger number of patients. We acknowledge that the study has some limitations: being a retrospective and limited to one cancer center. Nevertheless, our study contributes evidence that supports the observed findings in previous studies and allows the continuation of this investigation topic.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
DMK has provided Principal Investigator of clinical trials, advisory and speaker services to AstraZeneca, Merck Sharp & Dohme, Roche and Bristol-Myers Squibb. JMRM has provided advisory and speaker services to Ipsen, Bristol-Myers Squibb, Merck Sharp & Dohme and Novartis and received sponsorship for travel and expenses from Bayer. RDH has provided advisory services to Merck Sharp & Dohme, and received sponsorship for travel and expenses from Roche. The rest of authors declare no conflict of interest.
Informed Consent
As it was a retrospective analysis and data were de-identified, we did not contact patients nor asked for consents.
Author Contributions
All authors contributed equally to this paper, data analysis and interpretation, writing the manuscript. Rita Dorantes Heredia, JRM, and DMK provide oversight and leadership responsability for the research activity, planning and execution, including mentorship to the core of the team.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Znaor A, Laversanne M, Bray F. Less overdiagnosis of kidney cancer? an age-period-cohort analysis of incidence trends in 16 populations worldwide. Int J Cancer. 2017;141(5):925-932.
doi pubmed - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Sabath E. Kidney disease in Latin America: indigenous and immigrants, the forgotten population. Kidney Int. 2019;96(4):1038.
doi pubmed - Mittal K, Rini B. Kidney cancer in 2012: new frontiers in kidney cancer research. Nat Rev Urol. 2013;10(2):70-72.
doi pubmed - Reynoso-Noveron N, Mohar A. [Cancer in Mexico: recommendations for its control]. Salud Publica Mex. 2014;56(5):418-420.
doi pubmed - Graham J, Dudani S, Heng DYC. Prognostication in kidney cancer: recent advances and future directions. J Clin Oncol. 2018;36(36):3567-3573.
doi pubmed - Manola J, Royston P, Elson P, McCormack JB, Mazumdar M, Negrier S, Escudier B, et al. Prognostic model for survival in patients with metastatic renal cell carcinoma: results from the international kidney cancer working group. Clin Cancer Res. 2011;17(16):5443-5450.
doi pubmed - Ko JJ, Choueiri TK, Rini BI, Lee JL, Kroeger N, Srinivas S, Harshman LC, et al. First-, second-, third-line therapy for mRCC: benchmarks for trial design from the IMDC. Br J Cancer. 2014;110(8):1917-1922.
doi pubmed - Macfarlane R, Heng DY, Xie W, Knox JJ, McDermott DF, Rini BI, Kollmannsberger C, et al. The impact of kidney function on the outcome of metastatic renal cell carcinoma patients treated with vascular endothelial growth factor-targeted therapy. Cancer. 2012;118(2):365-370.
doi pubmed - Speed JM, Trinh QD, Choueiri TK, Sun M. Recurrence in localized renal cell carcinoma: a systematic review of contemporary data. Curr Urol Rep. 2017;18(2):15.
doi pubmed - Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530-2540.
doi pubmed - Motzer RJ, Murphy BA, Mazumdar M, et al. Randomized phase III trial of interferon alfa-2a (IFN) versus IFN plus 13-cis-retinoic acid (CRA) in patients (pts) with advanced renal cell carcinoma (RCC). Proc Am Soc Clin Oncol. 1999;18(16):2972-2980.
doi pubmed - Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794-5799.
doi pubmed - Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141-148.
doi - Fiala O, Finek J, Poprach A, Melichar B, Kopecky J, Zemanova M, Kopeckova K, et al. Outcomes according to MSKCC risk score with focus on the intermediate-risk group in metastatic renal cell carcinoma patients treated with first-line sunitinib: a retrospective analysis of 2390 patients. Cancers (Basel). 2020;12(4):808.
doi pubmed - Klatte T, Stewart GD. Disagreement in risk groups for metastatic renal cancer. Nat Rev Urol. 2019;16(6):332-333.
doi pubmed - Hatakeyama S, Tanaka T, Ikehata Y, Fujita N, Yamamoto H, Yoneyama T, et al. Impact of disagreement between the IMDC and MSKCC risk groups on prognosis in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology. 2019;37(7_suppl):556-556.
doi - Okita K, Hatakeyama S, Tanaka T, Ikehata Y, Tanaka T, Fujita N, Ishibashi Y, et al. Impact of disagreement between two risk group models on prognosis in patients with metastatic renal-cell carcinoma. Clin Genitourin Cancer. 2019;17(3):e440-e446.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


