| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 13, Number 1, February 2022, pages 1-7
Advances in the Treatment of Mucoepidermoid Carcinoma
Srikar Samaa, Takefumi Komiyab, Achuta Kumar Guddatia, c
aDivision of Hematology/Oncology, Georgia Cancer Center, Augusta University, Augusta, GA 30912, USA
bMedical Oncology, Parkview Cancer Institute, 11050 Parkview Circle, Fort Wayne, IN 46845, USA
cCorresponding Author: Achuta Kumar Guddati, Division of Hematology/Oncology, Georgia Cancer Center, Augusta University, Augusta, GA 30912, USA
Manuscript submitted August 18, 2021, accepted October 1, 2021, published online December 8, 2021
Short title: Advances in the Treatment of MEC
doi: https://doi.org/10.14740/wjon1412
| Abstract | ▴Top |
Mucoepidermoid carcinoma (MEC) represents 10-15% of salivary neoplasms. Due to their low incidence, it is challenging to conduct clinical trials and develop treatment guidelines. Although surgery is the most common approach for a resectable tumor, various treatment options such as chemotherapy, radiotherapy, and immunotherapy have been investigated. There is a need to implement a standardized treatment protocol to effectively manage MEC as it is a common histological subtype. Furthermore, it has become essential to assess chromosomal and genetic abnormalities recently identified with MEC, including alterations of CDKN2A, TP53, CDKN2B, BAP1, etc. These mutations are involved in the transformation of low-grade tumors to high-grade tumors, presenting a vital tool for evaluating the aggressive behavior of this carcinoma. Detailed immunohistochemical and translocation studies can help develop targeted therapies and monitor treatment response. Therefore, biomarker-driven research will immensely improve the outcome, especially in advanced cases. Based on thorough histology and chromosomal translocations, a more personalized treatment plan can improve the overall disease outcome. The purpose of this article is to elaborate on the current treatment advancements, particularly chemotherapy and targeted therapy, as an effective treatment modality for the management of MEC and highlight the comparison with traditional treatment approaches.
Keywords: Mucoepidermoid carcinoma; Salivary glands; Chemotherapy; Targeted therapy
| Introduction | ▴Top |
Mucoepidermoid carcinoma (MEC) is the most common malignant salivary gland tumor, accounting for 10-15% of all salivary gland tumors and one-third of all salivary gland malignancies [1, 2]. It is believed to arise from reserve cells of excretory ducts that are pluripotent in nature [3]. MEC has a wide age range (15 - 86 years, median 49 years), and a slight female predominance was observed [4, 5]. MEC commonly occurs in the parotid gland, with the submandibular and sublingual glands being the subsequent two common sites [6]. MEC diagnosis carries an excellent prognosis in adults, with an approximately 5-year survival rate of 98.8% in low grade, 97.4% in the intermediate grade, and about 67% for high-grade tumors [6]. MEC commonly presents as a painless swelling with pressure; however, symptoms vary with tumor size and site [7]. High-grade tumors tend to metastasize to local lymph nodes, but rarely distant metastases can also occur [8]. However, even low-grade MEC has also been shown to metastasize [9].
The earliest salivary gland cancers stage 0 (carcinoma in situ), and then stages range from I through IV (Table 1). MEC is relatively rare with a variable presentation; thus, different opinions have emerged about the treatment plan [4, 9, 10]. Salivary gland tumors are like any other salivary gland malignancy; the mainstay treatment for MEC is surgical resection with disease-free margins [11]. When there is perineural invasion, lymph node involvement, advanced high-grade tumors, positive margins after resection, and extra-glandular extension, adjuvant radiotherapy is recommended [12, 13]. Combined chemoradiotherapy showed better regional control, but no difference was found between the overall survival rate and patients receiving radiotherapy alone [14]. Systemic therapies have shown no evidence to improve survival; therefore, they should be used as palliative treatment for cancer-related symptoms relief or in rapid progression of the disease [15-17]. Clinical trials are being conducted to find out the efficacy of novel drugs. The purpose of writing this paper is to review the standard treatment and latest advances in systemic therapies in the treatment of MEC and bring out the importance of the role of targeted therapies.
 Click to view | Table 1. Staging of Salivary Gland Cancers |
| Standard Therapy | ▴Top |
Malignant salivary gland tumors are best removed through surgery. For the high-grade tumor, complete resection with negative surgical margin and lymph node dissection is indicated (Fig. 1). The goal should be to effectively plan the removal of most of the tumor without damaging the facial nerve. Intraoperative assessment of the facial nerve is necessary to identify early nerve invasion, which is not detected on preoperative imaging. The most critical factor in determining the prolonged outcome and disease-specific survival is locoregional disease control. Postoperative radiotherapy enhances locoregional control and is typically reserved for cancers with high-risk characteristics, such as close or positive surgical margins, nodal metastases, extracapsular spread (ECS), perineural invasion, lymphovascular invasion, advanced tumor (T) stage, and high-grade histopathology [17].
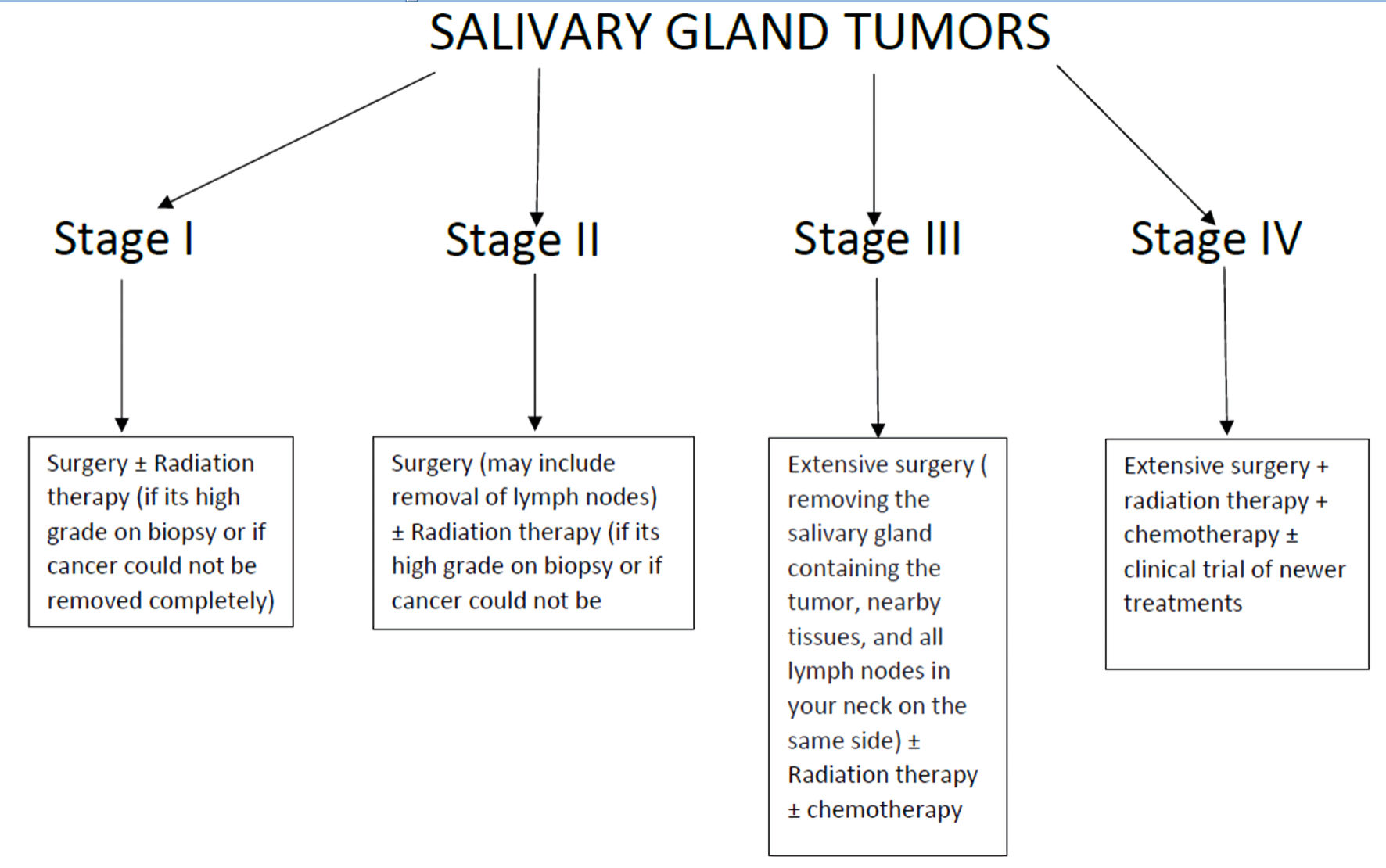 Click for large image | Figure 1. Stage-based management strategy for mucoepidermoid carcinoma. |
In the case of deep lobe and recurrent cancers, radiation may also be an option. High doses of greater than 60 Gy are necessary to achieve maximal local tumor control and other therapies. Radiation therapy, alone or in combination with chemotherapy, may be used to treat medically or technically unresectable tumors definitively at a dose of 66 Gy or greater [18].
| Clinical Trials | ▴Top |
Clinical trials were summarized in Table 2 [19-25].
 Click to view | Table 2. Various Clinical Trials Conducted in Treating Mucoepidermoid Carcinoma of the Salivary Gland |
Chemotherapy
Cisplatin plus vinorelbine (VNB)
VNB, when used alone in adenocarcinoma and adenoid cystic carcinoma, has a moderate activity. It binds to microtubular proteins in the mitotic spindle, and its mechanism of action is different from 5-FU, cisplatin, and anthracycline/mitoxantrone. Airoldi et al conducted a study, the average duration of partial response for patients treated with cisplatin plus VNB was 7.5 months (range, 3 - 11 months), the median stable disease duration was 5 months (range, 3 - 8 months), the median time to disease progression was 7 months, and the median overall survival duration was 11 months (range, 3 - 29 months) [19]. VNB alone is less effective than the combination therapy that involves cisplatin and VNB with a 19% complete response rate and some long-term survivors. The study’s poor results could be due to the high percentage of adenoid cystic carcinoma, and also the patients had been treated heavily previously. Palliation of pain and local disease control was observed, despite the absence of an apparent survival benefit.
Paclitaxel
In a phase II evaluation of single-agent paclitaxel, it was reported a moderate activity in mucoepidermoid carcinoma [20]. Among the 14 eligible patients with MEC who received paclitaxel, the median age was 67 years (range, 53 - 83 years), the 1-, 3-, 5-year survival rates were 0.57, 0.11, 0.00, respectively; it took more than 6 months for progression in four MEC patients, and partial response was noted in three patients. Common toxic events reported in this study were leukopenia (in six MEC patients) and granulocytopenia (in seven MEC patients). Gilbert et al [20] observed a variation in paclitaxel sensitivity among the histological subtypes: adenoid cystic carcinoma compared with mucoepidermoid and adenocarcinoma.
Docetaxel
Raguse et al determined the mechanism of docetaxel in four patients with high-grade mucoepidermoid cancer of the major salivary glands. After six cycles, complete remission was noticed in two patients, and partial remission was seen in the other two patients [21]. Docetaxel has shown excellent antitumor activity in squamous cell carcinoma of the head and neck [26]. Thus, this drug seems like a logical alternative in salivary gland tumors, but it needs further investigation with large sample size.
Monoclonal antibody
Trastuzumab
Trastuzumab is considered a monoclonal antibody that is effective against the human epidermal growth factor receptor 2 (HER2/neu) receptors. Many studies suggest amplification/overexpression of HER2/neu in mucoepidermoid carcinomas [27-30]. According to Lagha et al, this rate ranged from 0% to 38% [15]. Haddad et al [22] conducted a phase II trial using Herceptin (trastuzumab) on 14 patients having overexpressed HER2/neu in their salivary gland tumors. Among the three patients with MEC, partial response was seen only in one patient, which lasted more than 2 years. Herceptin as a single agent has a limited response. Hence it should be combined with other agents for a better therapeutic activity.
Targeted therapies
Sorafenib
In a phase II trial conducted on 37 adult patients with recurrent and malignant salivary gland cancer, Locati et al reported that sorafenib was included among the first antiangiogenic agents mainly effective in recurrent and metastatic salivary gland carcinoma [23]. There is a rapid decrease in disease progression in two patients with this disease because MEC showed the highest angiogenic activity with increased expression of vascular endothelial growth factor (VEGF) and ANG2 [31-33]. The median duration of response was 3.3 months (range, 1.2 - 20.2 months), the median progression-free survival was 4.2 months, and prolonged stabilization (> 6 months) was observed more commonly in non-adenoid cystic carcinoma patients. Tumor necrosis with cavitation occurred in one patient with a high-grade MEC, which is an expected effect of such agents.
Nintedanib
Nintedanib is an angiokinase inhibitor that attacks proangiogenic pathways mediated by VEGF receptor (VEGFR), fibroblast growth factor receptor (FGFR), and platelet-derived growth factor receptor (PDGFR). In a single-arm phase II trial done by Kim et al, 20 patients were enrolled, of which two patients (10%) had mucoepidermoid carcinoma [24]. There were no partial responders; in 15 patients (75%), the stable disease/disease-control rate was recorded. The median duration of stable disease was 8.2 months (range, 1.76 - 12.36 months), and the progression-free survival rate at 6 months was 60%. Most patients are well tolerated after nintedanib use, and dose reduction was needed only in four due to aspartate transaminase (AST)/alanine aminotransferase (ALT) elevations. Other side effects include diarrhea (35%) and nausea (25%). This study has limitations since all the subtypes of salivary gland cancers (SGCs) were included in this trial. Further investigation for each specific histological type is required.
Lapatinib
Lapatinib inhibits both epidermal growth factor receptor (EGFR) and receptor tyrosine-protein kinase (erbB2) receptors. When erbB2 is overexpressed in MEC, there is an increased risk of death compared to patients with little or no expression of erbB2 [34]. According to Lujan et al, the EGFR pathway is activated in high-grade MECs with aggressive behavior [34]. In a phase II study done by Agulnik et al [25], no objective response was noticed in the two MEC patients; but the stable disease (> 6 months) was observed in 36% of all the eligible patients [25]. Thus, the antitumor effect of lapatinib is mainly cytostatic, and more studies need to be done regarding the use of lapatinib in combination with other targeted molecular therapies.
ANA-12
ANA-12 is a tyrosine receptor kinase B (TrkB) inhibitor. The brain-derived neutropenic factor (BDNF) is a growth factor that binds to TrkB and activates downstream pathways like PI3K/Akt, which has a crucial role in tumorigenesis [35]. BDNF and TrkB expression are associated with perineural invasion in high-grade MEC, a poor prognostic factor [36, 37]. According to Wagner et al, TrkB inhibition decreases invasion and delayed migration, thus decreasing the in vitro survival of MEC cells [37]. However, this study also reported that the combination therapy of cisplatin and ANA-12 caused recovery and accumulation of cancer stem cells (CSC), indicating that there must be a limiting factor. The latest research has shown that the CSC can start the growth of new tumors and interfere with conventional therapy in MEC [38, 39].
Vorinostat
Vorinostat is a histone deacetylase inhibitor (HDACi). Recent evidence suggests that acetylation of chromatin by drugs in squamous cell carcinoma of the head and neck (HNSCC) can cause drastic phenotypic changes in cancer cells like the destruction of tumorspheres [40, 41]. According to Almeida et al, HDACi can avoid resistance to chemotherapy in HNSCC tumors [41]. Guimaraes et al demonstrated the impact of HDACi and cisplatin on CSCs taken from two MEC cell lines [38]. The results show that cisplatin is not effective against CSCs. Secondly, they found out that there can be a depletion of CSCs even at extremely low concentrations of HDACi. Furthermore, administration of HDACi before cisplatin depleted CSCs and thus sensitized the tumor cells to cisplatin. In addition, this pre-administration of HDACi reduced the amount of cisplatin needed to achieve the half-maximal inhibitory concentration (IC50). This result, in particular, is significant because we can pre-treat the patients with HDACi who fail the initial chemotherapy due to high toxicity [42].
| Discussion | ▴Top |
International Agency for Research on Cancer presents a report showing that salivary gland carcinomas will increase by more than 55% in the next 22 years, indicating the need for extensive research [43]. Currently, surgical management and adjuvant radiotherapy are the best options for achieving disease control because conventional chemotherapies are ineffective against this disease because of resistance [44-47]. The recent shift towards targeted therapies involving signaling pathways considering molecular signatures was a much needed change [48] (Table 3).
 Click to view | Table 3. Oncogenes and Its Potential Therapeutic Targets in Treating Mucoepidermoid Carcinoma |
Recent research has demonstrated many molecular targets in MEC. CRTC1/MAML2 fusion in a low-grade tumor is associated with a better prognosis [49-53]. Some evidence suggests HER2, EGFR or MUC1 is expressed more in high-grade tumors, which indicate a poor prognosis [54]. In addition, according to the reports, the presence of markers such as Ki-67, CEA, p53, and c-erbB-2 is directly associated with a patient’s survival with MEC [55]. The t(11;19) (q21;p12-13) chromosomal translocation is the most frequently detected translocation (27%) in MEC [56]. It has been shown that the expression of the fusion protein MECT1/MAML2 activated the cAMP/CREB pathway, is essential for tumor cell growth and is an attractive target for this cancer [57, 58]. Notably, MEC can occur in other organs, but this review will pertain only to salivary gland cancers [59]. There are other mutations/genomic changes which are site agnostic and for whom therapeutic agents are available regardless of the tumor histology, pembrolizumab for microsatellite instability-high (MSI) tumors and larotrectinib/entrectinib for neurotrophin receptor tyrosine kinase (NTRK) mutations [60, 61].
| Conclusions | ▴Top |
The role of systemic therapies in managing such advanced, recurrent and metastatic tumors still needs to be defined. In fact, most anticancer drugs are active against rapidly proliferating cells; thus, the slow growth of SGC could explain the poor results. Treatment choice should be dictated by histologic subtypes, patient characteristics and comorbidities, toxicity and cost of drugs. Further clinical trials with new drugs, new targeted therapies and new combinations to determine better systemic treatment are required.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no competing interests.
Author Contributions
Srikar Sama, Takefumi Komiya and Achuta Kumar Guddati: study design, data analysis and manuscript writing. All authors have read the manuscript and agree to the content.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Pires FR, Pringle GA, de Almeida OP, Chen SY. Intra-oral minor salivary gland tumors: a clinicopathological study of 546 cases. Oral Oncol. 2007;43(5):463-470.
doi pubmed - Xu W, Wang Y, Qi X, Xie J, Wei Z, Yin X, Wang Z, et al. Prognostic factors of palatal mucoepidermoid carcinoma: a retrospective analysis based on a double-center study. Sci Rep. 2017;7:43907.
doi pubmed - Batsakis JG. Salivary gland neoplasia: an outcome of modified morphogenesis and cytodifferentiation. Oral Surg Oral Med Oral Pathol. 1980;49(3):229-232.
doi - Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835-845.
doi pubmed - Rapidis AD, Givalos N, Gakiopoulou H, Stavrianos SD, Faratzis G, Lagogiannis GA, Katsilieris I, et al. Mucoepidermoid carcinoma of the salivary glands. Review of the literature and clinicopathological analysis of 18 patients. Oral Oncol. 2007;43(2):130-136.
doi pubmed - Mucoepidermoid carcinoma. 2021. Available from: https://www.pathologyoutlines.com/topic/salivaryglandsMEC.html.
- Peraza A, Gomez R, Beltran J, Amarista FJ. Mucoepidermoid carcinoma. An update and review of the literature. J Stomatol Oral Maxillofac Surg. 2020;121(6):713-720.
doi pubmed - Popalzai MJ, Aoun N, Baz W, Mourad M, Forte F, Friscia P. A case of metastatic mucoepidermoid carcinoma complicated by resistant hypercalcemia. Clin Med Insights Oncol. 2011;5:83-87.
doi pubmed - Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82(7):1217-1224.
doi - Nascimento AG, Amaral LP, Prado LA, Kligerman J, Silveira TR. Mucoepidermoid carcinoma of salivary glands: a clinicopathologic study of 46 cases. Head Neck Surg. 1986;8(6):409-417.
doi pubmed - Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg. 2005;63(6):805-810.
doi pubmed - Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer. 2005;103(12):2544-2550.
doi pubmed - Roh JL, Choi SH, Lee SW, Cho KJ, Nam SY, Kim SY. Carcinomas arising in the submandibular gland: high propensity for systemic failure. J Surg Oncol. 2008;97(6):533-537.
doi pubmed - de Souza LB, de Oliveira LC, Nonaka CFW, Lopes M, Pinto LP, Queiroz LMG. Immunoexpression of GLUT-1 and angiogenic index in pleomorphic adenomas, adenoid cystic carcinomas, and mucoepidermoid carcinomas of the salivary glands. Eur Arch Otorhinolaryngol. 2017;274(6):2549-2556.
doi pubmed - Lagha A, Chraiet N, Ayadi M, Krimi S, Allani B, Rifi H, Raies H, et al. Systemic therapy in the management of metastatic or advanced salivary gland cancers. Oral Oncol. 2012;48(10):948-957.
doi pubmed - Limaye SA, Posner MR, Krane JF, Fonfria M, Lorch JH, Dillon DA, Shreenivas AV, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. 2013;18(3):294-300.
doi pubmed - Lewis AG, Tong T, Maghami E. Diagnosis and Management of Malignant Salivary Gland Tumors of the Parotid Gland. Otolaryngol Clin North Am. 2016;49(2):343-380.
doi pubmed - Pederson AW, Salama JK, Haraf DJ, Witt ME, Stenson KM, Portugal L, Seiwert T, et al. Adjuvant chemoradiotherapy for locoregionally advanced and high-risk salivary gland malignancies. Head Neck Oncol. 2011;3:31.
doi pubmed - Airoldi M, Pedani F, Succo G, Gabriele AM, Ragona R, Marchionatti S, Bumma C. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer. 2001;91(3):541-547.
doi - Gilbert J, Li Y, Pinto HA, Jennings T, Kies MS, Silverman P, Forastiere AA. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2006;28(3):197-204.
doi pubmed - Raguse JD, Gath HJ, Bier J, Riess H, Oettle H. Docetaxel (Taxotere) in recurrent high grade mucoepidermoid carcinoma of the major salivary glands. Oral Oncology Extra. 2004;40(1):5-7.
doi - Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, Weeks L, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39(7):724-727.
doi - Locati LD, Perrone F, Cortelazzi B, Bergamini C, Bossi P, Civelli E, Morosi C, et al. A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: Translational analyses and clinical impact. Eur J Cancer. 2016;69:158-165.
doi pubmed - Kim Y, Lee SJ, Lee JY, Lee SH, Sun JM, Park K, An HJ, et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: A multicenter phase 2 study (Korean Cancer Study Group HN14-01). Cancer. 2017;123(11):1958-1964.
doi pubmed - Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, Winquist E, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25(25):3978-3984.
doi pubmed - Catimel G, Verweij J, Mattijssen V, Hanauske A, Piccart M, Wanders J, Franklin H, et al. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials Group. Ann Oncol. 1994;5(6):533-537.
doi pubmed - Nakano T, Yamamoto H, Hashimoto K, Tamiya S, Shiratsuchi H, Nakashima T, Nishiyama K, et al. HER2 and EGFR gene copy number alterations are predominant in high-grade salivary mucoepidermoid carcinoma irrespective of MAML2 fusion status. Histopathology. 2013;63(3):378-392.
doi pubmed - Nguyen LH, Black MJ, Hier M, Chauvin P, Rochon L. HER2/neu and Ki-67 as prognostic indicators in mucoepidermoid carcinoma of salivary glands. J Otolaryngol. 2003;32(5):328-331.
doi pubmed - Alotaibi AM, Alqarni MA, Alnobi A, Tarakji B. Human epidermal growth factor receptor 2 (HER2/neu) in salivary gland carcinomas: a review of literature. J Clin Diagn Res. 2015;9(2):ZE04-08.
doi pubmed - Kurzrock R, Bowles DW, Kang H, Meric-Bernstam F, Hainsworth J, Spigel DR, Bose R, et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: results from MyPathway, a phase IIa multiple basket study. Ann Oncol. 2020;31(3):412-421.
doi pubmed - Cardoso SV, Souza KC, Faria PR, Eisenberg AL, Dias FL, Loyola AM. Assessment of angiogenesis by CD105 antigen in epithelial salivary gland neoplasms with diverse metastatic behavior. BMC Cancer. 2009;9:391.
doi pubmed - Gleber-Netto FO, Florencio TN, de Sousa SF, Abreu MH, Mendonca EF, Aguiar MC. Angiogenesis and lymphangiogenesis in mucoepidermoid carcinoma of minor salivary glands. J Oral Pathol Med. 2012;41(8):603-609.
doi pubmed - Demasi AP, Silva CA, Silva AD, Furuse C, Soares AB, Altemani A, Napimoga MH, et al. Expression of the vascular endothelial growth factor and angiopoietins in mucoepidermoid carcinoma of salivary gland. Head Neck Pathol. 2012;6(1):10-15.
doi pubmed - Lujan B, Hakim S, Moyano S, Nadal A, Caballero M, Diaz A, Valera A, et al. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br J Cancer. 2010;103(4):510-516.
doi pubmed - de Moraes JK, Wagner VP, Fonseca FP, Vargas PA, de Farias CB, Roesler R, Martins MD. Uncovering the role of brain-derived neurotrophic factor/tyrosine kinase receptor B signaling in head and neck malignancies. J Oral Pathol Med. 2018;47(3):221-227.
doi pubmed - McHugh CH, Roberts DB, El-Naggar AK, Hanna EY, Garden AS, Kies MS, Weber RS, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer. 2012;118(16):3928-3936.
doi pubmed - Wagner VP, Martins MD, Amoura E, Zanella VG, Roesler R, de Farias CB, Bingle CD, et al. TrkB-targeted therapy for mucoepidermoid carcinoma. Biomedicines. 2020;8(12):531.
doi pubmed - Guimaraes DM, Almeida LO, Martins MD, Warner KA, Silva AR, Vargas PA, Nunes FD, et al. Sensitizing mucoepidermoid carcinomas to chemotherapy by targeted disruption of cancer stem cells. Oncotarget. 2016;7(27):42447-42460.
doi pubmed - Adams A, Warner K, Pearson AT, Zhang Z, Kim HS, Mochizuki D, Basura G, et al. ALDH/CD44 identifies uniquely tumorigenic cancer stem cells in salivary gland mucoepidermoid carcinomas. Oncotarget. 2015;6(29):26633-26650.
doi pubmed - Giudice FS, Pinto DS, Jr., Nor JE, Squarize CH, Castilho RM. Inhibition of histone deacetylase impacts cancer stem cells and induces epithelial-mesenchyme transition of head and neck cancer. PLoS One. 2013;8(3):e58672.
doi pubmed - Almeida LO, Abrahao AC, Rosselli-Murai LK, Giudice FS, Zagni C, Leopoldino AM, Squarize CH, et al. NFkappaB mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC). FEBS Open Bio. 2014;4:96-104.
doi pubmed - Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8(5):368-379.
doi - Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953.
doi pubmed - Kaplan MJ, Johns ME, Cantrell RW. Chemotherapy for salivary gland cancer. Otolaryngol Head Neck Surg. 1986;95(2):165-170.
doi pubmed - Pires FR, de Almeida OP, de Araujo VC, Kowalski LP. Prognostic factors in head and neck mucoepidermoid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(2):174-180.
doi pubmed - Grisanti S, Amoroso V, Buglione M, Rosati A, Gatta R, Pizzocaro C, Ferrari VD, et al. Cetuximab in the treatment of metastatic mucoepidermoid carcinoma of the salivary glands: a case report and review of literature. J Med Case Rep. 2008;2:320.
doi pubmed - Guzzo M, Andreola S, Sirizzotti G, Cantu G. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002;9(7):688-695.
doi pubmed - Wagner VP, Martins MD, Martins MAT, Almeida LO, Warner KA, Nor JE, Squarize CH, et al. Targeting histone deacetylase and NFkappaB signaling as a novel therapy for Mucoepidermoid Carcinomas. Sci Rep. 2018;8(1):2065.
doi pubmed - Behboudi A, Enlund F, Winnes M, Andren Y, Nordkvist A, Leivo I, Flaberg E, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45(5):470-481.
doi pubmed - Okabe M, Miyabe S, Nagatsuka H, Terada A, Hanai N, Yokoi M, Shimozato K, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12(13):3902-3907.
doi pubmed - Jee KJ, Persson M, Heikinheimo K, Passador-Santos F, Aro K, Knuutila S, Odell EW, et al. Genomic profiles and CRTC1-MAML2 fusion distinguish different subtypes of mucoepidermoid carcinoma. Mod Pathol. 2013;26(2):213-222.
doi pubmed - Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin's tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46(7):708-715.
doi pubmed - Cipriani NA, Lusardi JJ, McElherne J, Pearson AT, Olivas AD, Fitzpatrick C, Lingen MW, et al. Mucoepidermoid carcinoma: a comparison of histologic grading systems and relationship to MAML2 rearrangement and prognosis. Am J Surg Pathol. 2019;43(7):885-897.
doi pubmed - Cros J, Sbidian E, Hans S, Roussel H, Scotte F, Tartour E, Brasnu D, et al. Expression and mutational status of treatment-relevant targets and key oncogenes in 123 malignant salivary gland tumours. Ann Oncol. 2013;24(10):2624-2629.
doi pubmed - Lopes MA, da Cruz Perez DE, de Abreu Alves F, de Almeida OP, Kowalski LP. Clinicopathologic and immunohistochemical study of intraoral mucoepidermoid carcinoma. Otolaryngol Head Neck Surg. 2006;134(4):622-626.
doi pubmed - Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O'Neil K, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208-213.
doi pubmed - Komiya T, Park Y, Modi S, Coxon AB, Oh H, Kaye FJ. Sustained expression of Mect1-Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene. 2006;25(45):6128-6132.
doi pubmed - Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA, Kirsch IR, et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res. 2005;65(16):7137-7144.
doi pubmed - Komiya T, Perez RP, Yamamoto S, Neupane P. Primary lung mucoepidermoid carcinoma: analysis of prognostic factors using surveillance, epidemiology and end results program. Clin Respir J. 2017;11(6):847-853.
doi pubmed - Diaz LA, Marabelle A, Delord JP, Shapira-Frommer R, Geva R, Peled N, Kim TW, et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J Clin Oncol. 2017;35(15_suppl):3071-3071.
doi - Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731-747.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


