| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Short Communication
Volume 13, Number 2, April 2022, pages 96-101
Increasing Trends in Mortality Rate Among Salivary Gland Tumors in Non-Geriatric African Americans
Varsha Chiruvellaa, William Blacka, Achuta Kumar Guddatib, c
aMedical College of Georgia, Augusta University, Augusta, GA 30909, USA
bDivision of Hematology/Oncology, Georgia Cancer Center, Augusta University, Augusta, GA 30909, USA
cCorresponding Author: Achuta Kumar Guddati, Division of Hematology/Oncology, Georgia Cancer Center, Augusta University, Augusta, GA 30909, USA
Manuscript submitted September 30, 2021, accepted January 21, 2022, published online March 12, 2022
Short title: Mortality in Salivary Gland Tumors
doi: https://doi.org/10.14740/wjon1420
| Abstract | ▴Top |
Background: The treatment of salivary gland tumors has not changed significantly in the past two decades. However, the increase in the geriatric population with these tumors poses a new challenge for their management. This study explores the incidence-based mortality trends in the geriatric and non-geriatric population for the time period of 2000 - 2014 and compares the trends between races.
Methods: Mortality data were extracted from the Surveillance, Epidemiology, and End Results (SEER) Database for the years 2000 - 2014. Incidence-based mortality for all stages of salivary gland tumors was queried and the results were grouped by age (geriatric vs. non-geriatric determined as 65 vs. below 65 years of age) and race (Caucasian/White, African American/Black, American Indian/Alaskan native and Asian/Pacific Islander). All stages and both genders were included in the analysis. T-test was used to determine statistically significant difference between various subgroups. Linearized trend lines were used to visualize the mortality trends between various subgroups (geriatric vs. non-geriatric and Caucasian vs. African American).
Results: Incidence-based mortality for salivary gland tumors has worsened since 2000 to 2014 for both geriatric and non-geriatric patients (P < 0.05). There was a statistically significant difference between these two groups in both Caucasian/White patients and African American/Black patients. Notably, the worst incidence-based mortality rates were noted in African American/Black non-geriatric patients followed by Caucasian/White non-geriatric patients. However, there was no statistical difference in incidence-based mortality between Caucasian/White patients and African American/Black geriatric patients.
Conclusions: The similarity in incidence-based mortality for geriatric patients with salivary gland tumors in both Caucasian/White patients and African American/Black groups suggests that the effects of race may not be pronounced in the elderly population. The high rate of incidence-based mortality in African American/Black non-geriatric patients may suggest environmental influence and warrants further study.
Keywords: Salivary gland tumor; Geriatric; Racial disparity; Incidence-based mortality
| Introduction | ▴Top |
Salivary gland cancers (SGCs) are a type of head and neck cancer (HNC) that can arise from any of the three major glands (parotid, sublingual, and submandibular) or from the various minor salivary glands. SGCs have uncertain epidemiology and comprise a relatively rare cancer, representing 3-10% of HNCs and less than 1% of all cancers in the USA [1, 2]. Salivary gland tumors are heterogeneous due to their origins in diverse glandular cell types and consist of at least 20 distinct histologic subtypes [3]. The annual estimated incidence of salivary gland tumors ranges from 0.4 to 13.5 cases per 100,000 individuals [4]. However, the incidence of SGCs has increased within the past four decades. It is estimated that the incidence of SGC incidence in the USA from 1975 to 2015 increased from 1.1 to 1.3 cases/100,000 individuals [5].
There have been previous studies that show patterns in demographic presentation of salivary gland tumors. Benign tumors tend to occur more frequently in women and are diagnosed at a younger age compared to malignant tumors, which present more commonly in males and are diagnosed at an older age [6, 7]. In a retrospective study of 11,007 patients with SGC by Russell et al, black individuals were shown to have poorer disease-specific survival compared to Whites [8]. The American Cancer Society also notes that older age is a risk factor for SGC, in addition to the male gender. In terms of patient age, a study by Bjorndal et al revealed that the younger (< 70 years), non-geriatric population had better, disease-specific survival when compared to elderly geriatric (> 70 years) [9]. This could be due to more advanced disease stage and high-grade histological subtypes of SGC present in the geriatric population. This finding challenges the incidence-based mortality trends found in this study. When comparing incidence between races, this study showed that disease-specific mortality rate was highest in black, non-geriatric patients. Given the discovered racial disparity and potential discrepancies or differences in SGC incidence-based mortality based on age, it is important to analyze mortality trends between various racial and aged populations. Current staging categorizes salivary gland carcinomas into either low or high grade, with intermediate grade demonstrating the most variability.
While there may be associations between race, age, and incidence-based mortality of SGC, histologic grade and stage of the tumor are still significant predictors of SGC prognosis. A retrospective study by Lima et al showed that five factors had significant negative influence on prognosis of malignant parotid gland tumors, of which age, stage, grade, and presence in lymph nodes were included. The 10-year disease-specific survival rate for SGC depends on the stage of the cancer. From stage I to IV, these rates were 97%, 81%, 56%, and 20%, respectively [10]. Furthermore, 20% of individuals with SGC will develop distant metastases, which corresponds with only 4.3 - 7.3 months of survival [11]. Those with high-grade salivary gland tumors are more likely to develop distant metastases and survive for a shorter time period compared to patients with low-grade cancer. It is furthermore important to consider gender as a factor in analyzing incidence-based mortality. A study by Cheung et al that queried Florida Cancer Registry and in-patient hospital data of SGC diagnoses showed that men were diagnosed with higher grade and advanced disease stage than women. Males also had poorer outcomes and 5-year survival rates than their female counterparts [12]. Given these data, it is imperative to also consider factors such as tumor stage, grade, gender and environmental factors when analyzing incidence-based mortality trends between different age and racial populations.
As there is discrepant literature regarding influences of race and age on mortality from SGC, there is a need to further assess the disease in context of these variables. Using data from the Surveillance, Epidemiology, and End Results (SEER) database from 2000 to 2014, this study aims to analyze incidence-based mortality trends from salivary gland tumors within geriatric and non-geriatric populations (65 years vs. below 65 years of age) and amongst Caucasian and African American races. A goal of this study is to assess demographic factors that impact SGC prognosis and corroborate or counter previous studies to better understand variables that influence mortality. The study thereby aims to focus individualized diagnostic and treatment strategies for certain populations.
| Materials and Methods | ▴Top |
Data source
The SEER database was used to extract mortality data from all stages of salivary gland tumors from 2000 to 2014. SEER is a database that is part of the National Cancer Institute (NCI) and a source of cancer incidence, survival, and statistics in the USA. SEER collects its data on patient demographics, primary tumor site, tumor morphology, and incidence from cancer registries spanning 34.6% of the US population.
Study population
Patients with all stages of salivary gland tumors were included in this study. Mortality data of patients of both genders and all ages (geriatric and non-geriatric) from 2000 to 2014 were studied. Patient demographic information such as gender, age, and race as well as tumor stage were included in analysis. This study limited analysis to patients of four racial/ethnic groups: Caucasian/White, African American/Black, American Indian/Alaskan native, and Asian/Pacific Islander. Patients were grouped as geriatric (> 65 years old) and non-geriatric (< 65 years old).
The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. This study was deemed to be exempt from IRB approval as it involved analysis of de-identified patients from a national database.
Outcomes
Incidence-based mortality rates from SGCs were calculated based on age (geriatric and non-geriatric) and the four racial/ethnic groups. Incidence-based mortality rates from SGC was defined as the number of deaths due to SGC among the total number of patient cases of SGC diagnosed in SEER regions.
Statistical analysis
T-tests were used to determine statistically significant differences between various age and racial subgroups listed above. Linearized trend lines were used to compare mortality trends between subgroups, such as geriatric vs. non-geriatric and Caucasian vs. African American. Statistical significance was defined as a P-value < 0.05.
| Results | ▴Top |
The average incidence-based mortality rates from 2000 to 2014 for salivary gland tumors for both races and age populations are shown in Table 1. There is a statistically significant difference between these two populations in both Caucasian/White patients and African American/Black patients. White and Black non-geriatric patients have higher incidence-based mortality rates than the geriatric population for both races (P < 0.05). Black non-geriatric patients also have a significantly higher mortality rate from SGC than White non-geriatric individuals (P < 0.05). Rates of mortality have also increased in both geriatric and non-geriatric patients since 2000 to 2014 (P < 0.05) (Figs. 1-4). Despite past reports that outcome from salivary gland tumors is poorer in elderly population, this study notes higher mortality rate in the non-geriatric population. There is no significant difference in mortality rates between White and Black geriatric patients with salivary gland tumors (P = 0.06).
 Click to view | Table 1. Average Incidence-Based Mortality Rates From 2000 to 2014 for Salivary Gland Tumors Based on Race and Age |
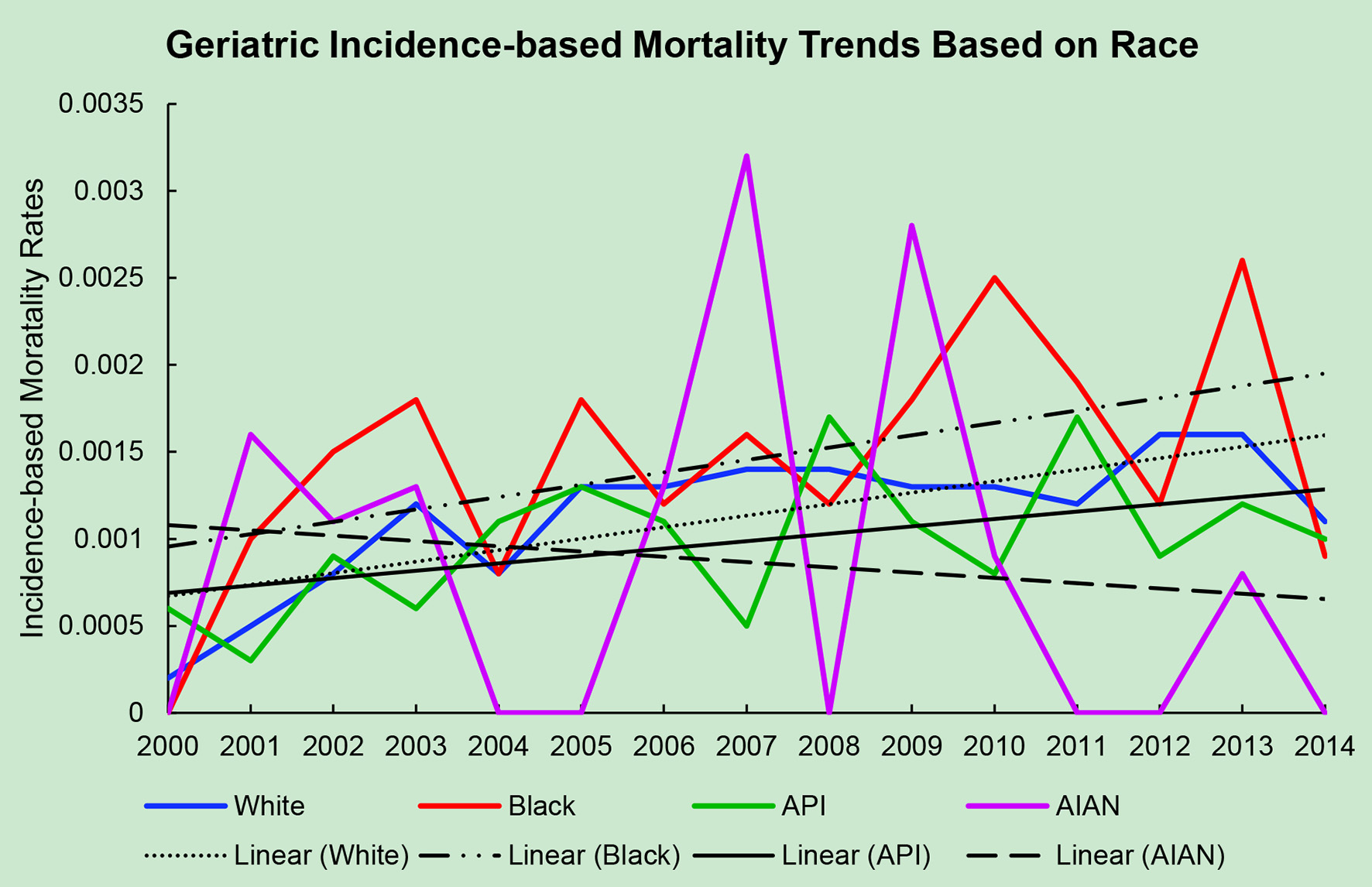 Click for large image | Figure 1. Geriatric incidence-based mortality trends relative to race based off mortality data via SEER from 2000 to 2014. API: Asian/Pacific Islander; AIAN: American Indian/Alaskan native. |
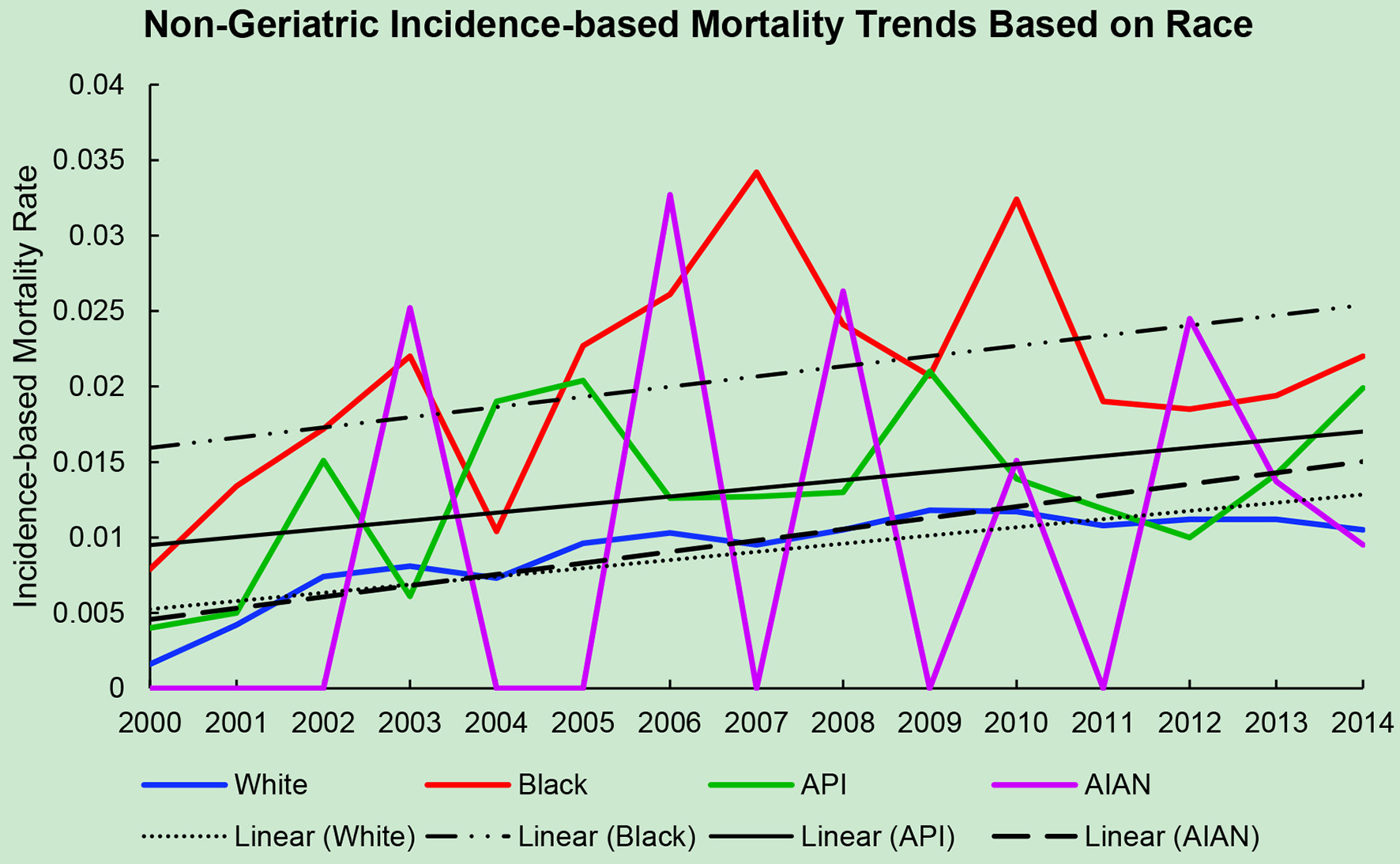 Click for large image | Figure 2. Non-geriatric incidence-based mortality trends relative to race based off mortality data via SEER from 2000 to 2014. API: Asian/Pacific Islander; AIAN: American Indian/Alaskan native. |
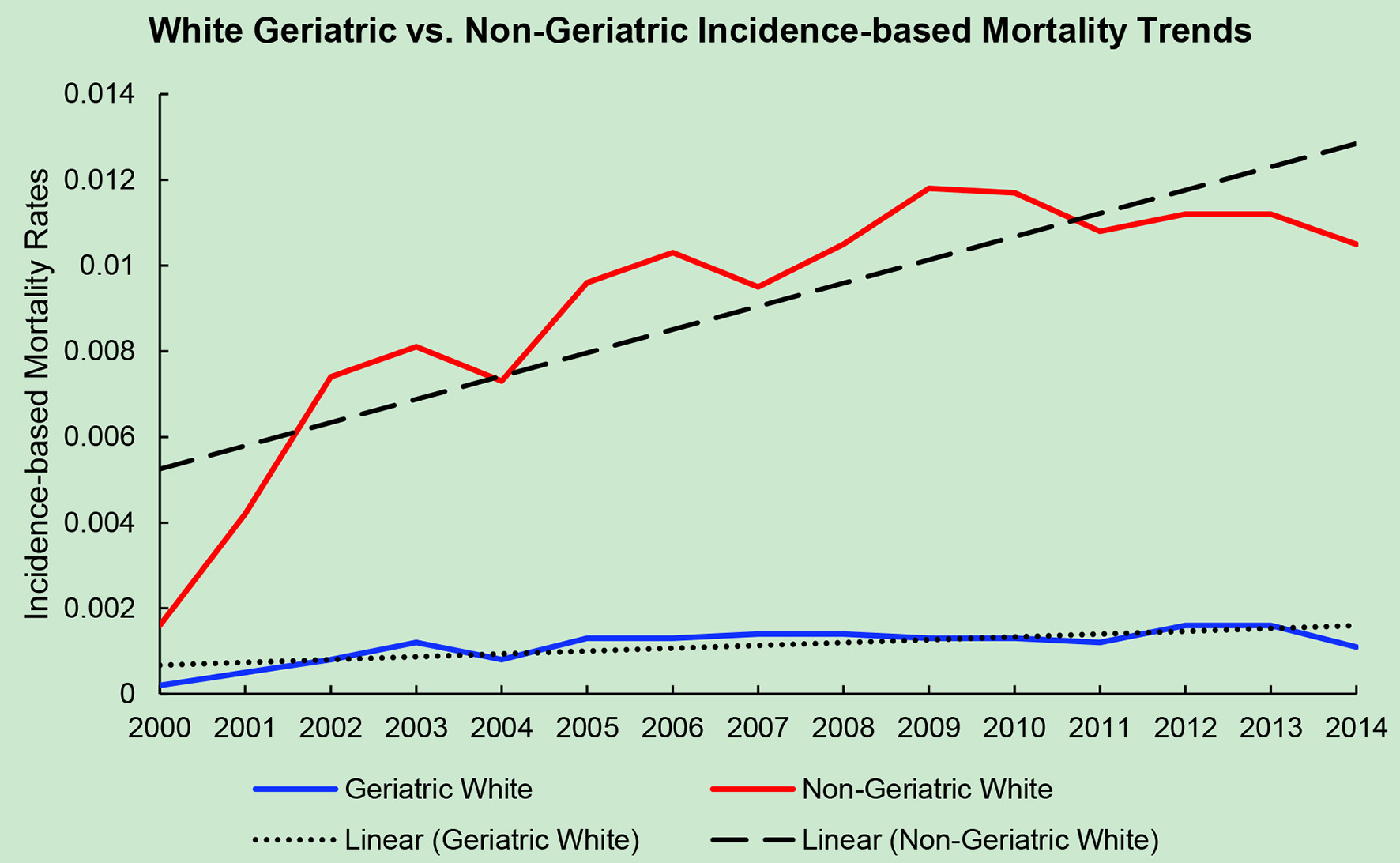 Click for large image | Figure 3. Geriatric versus non-geriatric incidence-based mortality trends among White population based off mortality data via SEER from 2000 to 2014. |
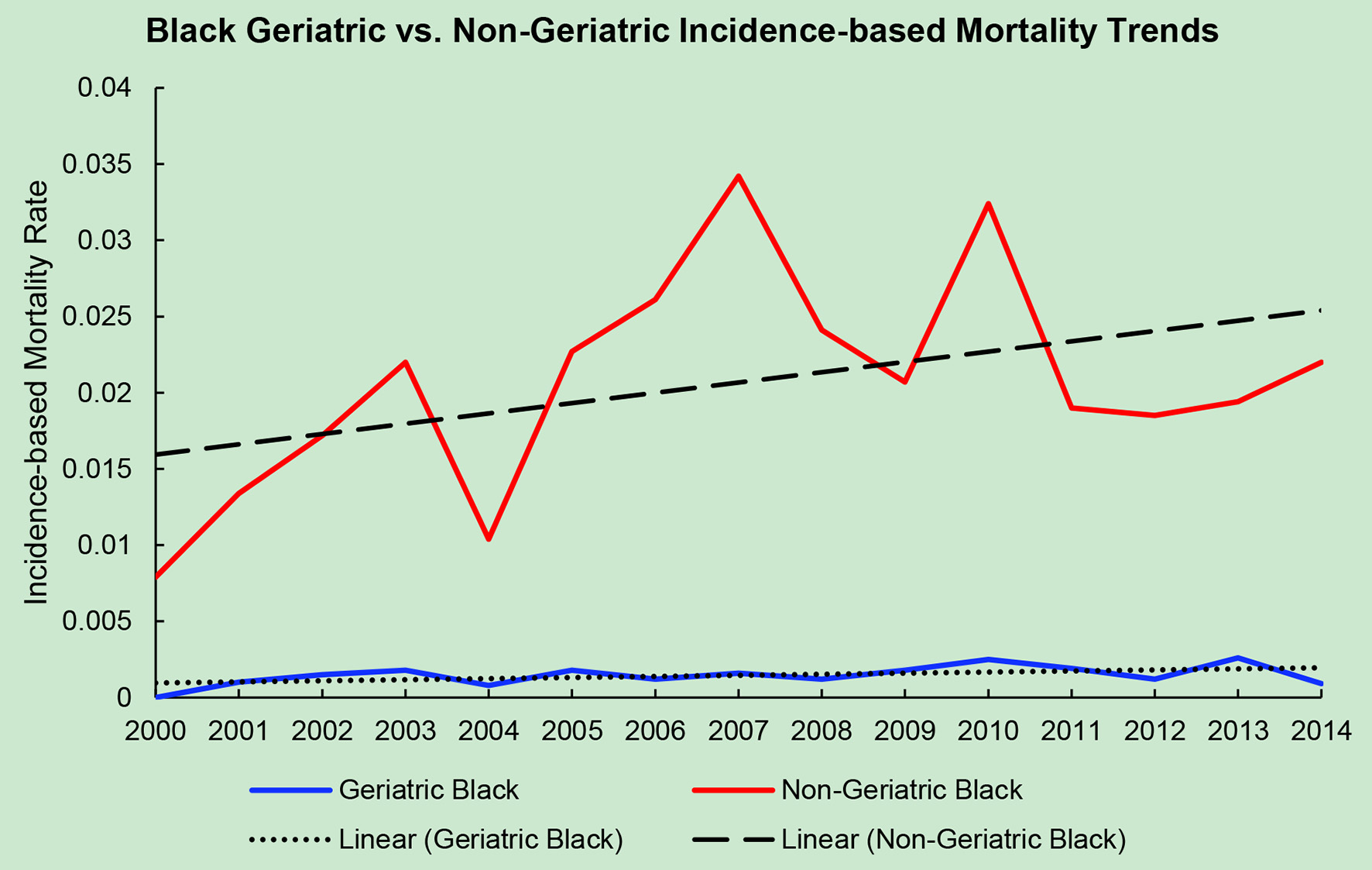 Click for large image | Figure 4. Geriatric versus non-geriatric incidence-based mortality trends among Black population based off mortality data via SEER from 2000 to 2014. |
| Discussion | ▴Top |
This study is a population-based analysis of trends in incidence-based mortality rates from all stages of salivary gland tumors in the USA. Patients with salivary gland tumors were identified utilizing the SEER database from 2000 to 2014. We evaluated mortality based on variables such as race, age, gender, tumor stage. This study is one of few that address the factor of age and incidence-based mortality from SGC. It also challenges past reports stating that mortality from salivary gland tumors is higher in the elderly population.
The most significant finding of this study are the increased mortality rates in the non-geriatric population of patients with salivary gland tumors in comparison to the geriatric population (non-geriatric age is < 65 years and geriatric age group is ≥ 65 years of age). This result challenges previous studies which established that the incidence of mortality from salivary gland tumors is higher in elderly individuals [9]. The variable levels of fitness, vulnerability, and high likelihood of pre-existing conditions in the geriatric population, in addition to the rapid expansion of this population over the years, warrant need for specialized geriatric oncologists. It is predicted that the number of individuals over 65 years will double from the turn of the century to 2050. As advanced age is a risk factor for cancer, a graying population indicates that diseases that target elderly individuals would become more prevalent. A dramatic increase in cancer incidence is reported in individuals over 65 years, with 60% of new malignant diagnoses and 70% of cancer deaths occurring in this population. Incidence of cancer in those over 65 is 10 times greater, and mortality from cancer is 16 times greater when compared to the non-geriatric population. Additionally, over 70% of mortalities associated with cancers such as prostate, bladder, colon, and lung occur in patients 65 and older [13, 14]. One explanation for the high mortality from HNC in elderly individuals is that geriatric patients may not be considered candidates for aggressive multimodality treatments that involve surgery, radiation, and/or chemotherapy due to high chances of comorbidities and concern over poor treatment and toxicity tolerance [15]. The interaction of comorbidities with old age could impact patient prognosis, quality of life, and management options. This issue is compounded by the fact that a large proportion of HNC patients present with locoregionally advanced (LA) stage III and IV cancer which in turn require multimodality treatment for survival. In fact, there is current debate on whether treatment options for geriatric population should be different than for younger individuals. Some reviews reject the proposal that age be a sole factor in determining whether curative therapy be undertaken for geriatric patients [16, 17].
One report states no significant difference in outcome from HNC between geriatric and non-geriatric patients, adding that postoperative complications occur no more frequently in the older population than the younger [17]. Concordant with this review, a study by Sesterhenn et al that analyzed postoperative complications from 24 patients over 75 years old suffering from squamous cell carcinoma (SCC) of the larynx showed no evidence of postoperative complications in patients when treated by transoral CO(2) laser microsurgery with curative intention [16]. A study by Zabrodsky et al reported acceptable complication rates in elderly patients treated with major surgery for HNC with meticulous assessment of pre-existing comorbidities [18]. Furthermore, a retrospective case-control study by Clayman et al showed no significant difference in perioperative or postoperative complications between patients 80 years and older with head and neck SCC and those 65 years and younger [19]. Bjorndal et al discovered that younger patients (< 70 years) with SGC have a significantly better disease-specific and recurrence-free survival than the elderly ones (> 70 years) [19]. The poorer prognosis of SGC in the geriatric population could be explained by more advanced disease stage, higher histological grade subtype, presence of comorbid conditions, and treatment challenges. Additionally, elderly individuals over 75 years old were found to have a statistically significant lower likelihood of receiving a resection surgery for major SGC when compared to patients younger than 75 years [20]. As such, the finding of this study that non-geriatric individuals, particularly of African American/Black race, have higher incidence-based mortality than geriatric individuals is a surprising report that warrants further investigation into whether this discrepancy is caused by genetic factors, systematic deficiencies, or some other contributor.
Studies such as this have shown that race and ethnicity influence prevalence, mortality rates and prognosis of SGC. This study found that mortality rates are higher in the African American/Black population in comparison to the Caucasian/White. Notably, the worst incidence-based mortality rates were noted in African American/Black non-geriatric patients followed by Caucasian/White non-geriatric patients. A large death certificate-based case-control study by Wilson et al studying SGC cases reported the greatest proportion of young deaths among African Americans (< 50 years) [21]. Russell et al similarly found that black patients had inferior outcomes compared to other races [8]. A retrospective study on patients using the SEER database from 2007 to 2014 showed that African Americans/Blacks had a less favorable health outcome and higher risk of mortality when compared to Caucasians/Whites, Asian/Pacific Islanders, and Hispanics [22]. In addition to age disparity, genetic/biologic factors, and comorbid conditions, socioeconomic factors and social inequalities such as access to healthcare play important roles in HNC survival. It is thus important to address both clinical and non-clinical factors when estimating disparities in overall survival and incidence-based mortality across racial groups. A SEER-Medicare linked retrospective study by Suzuki et al of 15,547 patients over 66 years diagnosed with HNC found that African American/Black patients had significantly inferior overall survival compared to all other racial/ethnic groups. It is important to note that this finding came despite similar treatments, comorbidities, age at diagnosis, and stage at presentation between African Americans/Black and Caucasians/White patients [23]. Independent of many confounding factors, African Americans may thus present with an overall inferior survival after diagnosis of HNC and a significantly worse outcome with malignancy of the oropharynx. African American patients have the worst prognosis of HNC, with age-adjusted mortality rate for Black men approximately twice that of White men [24].
This study has several limitations. This study has a retrospective design and uses a database which could contribute to inherent study bias. As such, we are unable to draw causal relationships from our data and must infer potential explanations for our results. We also acknowledge that there may be inconsistencies when comparing data between studies. For example, geriatric may be defined differently or may have differing classifications in various studies. This lack of consistency in classifying certain age groups can make comparisons difficult.
Conclusion
Using the national SEER database, this study analyzes the rates of incidence-based mortality from all stages of salivary gland tumors with respect to race/ethnicity and age of patients between the years 2000 and 2014. Overall, our study highlights a higher rate of incidence-based mortality in non-geriatric and African Americans compared to the geriatric and Caucasian populations. Our results imply that non-geriatric African Americans have the worst outcomes in terms of incidence-based mortality. This conclusion challenges previous literature on age-related mortality disparity in patients with salivary gland tumors. It also concurs with past studies that report African Americans have poorer prognoses than Caucasians after diagnoses of HNC, and particularly salivary gland carcinoma. Further investigation into possible environmental, occupational, and genetic factors and mechanisms that contribute to disparity in mortality rates in the non-geriatric population is warranted. This could gain a better understanding of health disparity and may impact individualized healthcare and patient outcome.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Conception and design: VC, AG. Analysis and interpretation: VC. Drafting and formatting: VC. Critical revision and final editing: VC, WB, AG.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Lawal AO, Adisa AO, Kolude B, Adeyemi BF. Malignant salivary gland tumours of the head and neck region: a single institutions review. Pan Afr Med J. 2015;20:121.
doi pubmed - American Cancer Society. What are the key statistics about salivary gland cancer? https://www.cancer.org/cancer/salivary-gland-cancer/about/what-is-key-statistics.html#written_by.
- Boukheris H, Curtis RE, Land CE, Dores GM. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2899-2906.
doi pubmed - Carlson ER, Schlieve T. Salivary gland malignancies. Oral Maxillofac Surg Clin North Am. 2019;31(1):125-144.
doi pubmed - Lin HH, Limesand KH, Ann DK. Current state of knowledge on salivary gland cancers. Crit Rev Oncog. 2018;23(3-4):139-151.
doi pubmed - Israel Y, Rachmiel A, Ziv G, Nagler R. Benign and malignant salivary gland tumors - clinical and demographic characteristics. Anticancer Res. 2016;36(8):4151-4154.
- Gao M, Hao Y, Huang MX, Ma DQ, Chen Y, Luo HY, Gao Y, et al. Salivary gland tumours in a northern Chinese population: a 50-year retrospective study of 7190 cases. Int J Oral Maxillofac Surg. 2017;46(3):343-349.
doi pubmed - Russell JL, Chen NW, Ortiz SJ, Schrank TP, Kuo YF, Resto VA. Racial and ethnic disparities in salivary gland cancer survival. JAMA Otolaryngol Head Neck Surg. 2014;140(6):504-512.
doi pubmed - Bjorndal K, Larsen SR, Therkildsen MH, Kristensen CA, Charabi B, Andersen E, Overgaard J, et al. Does age affect prognosis in salivary gland carcinoma patients? A national Danish study. Acta Oncol. 2016;55(Suppl 1):19-22.
doi pubmed - Lima RA, Tavares MR, Dias FL, Kligerman J, Nascimento MF, Barbosa MM, Cernea CR, et al. Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol Head Neck Surg. 2005;133(5):702-708.
doi pubmed - Schwentner I, Obrist P, Thumfart W, Sprinzl G. Distant metastasis of parotid gland tumors. Acta Otolaryngol. 2006;126(4):340-345.
doi pubmed - Cheung MC, Franzmann E, Sola JE, Pincus DJ, Koniaris LG. A comprehensive analysis of parotid and salivary gland cancer: worse outcomes for male gender. J Surg Res. 2011;171(1):151-158.
doi pubmed - Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg LX. SEER cancer statistics review, 1973-1998. National Institute of Health. 2000.
- Yancik R, ME H. Exploring the role of cancer centers for integrating aging and cancer research. NIA/NCI Report of the Cancer Center Workshop (June 13-15, 2001). 2002.
- Siddiqui F, Gwede CK. Head and neck cancer in the elderly population. Semin Radiat Oncol. 2012;22(4):321-333.
doi pubmed - Sesterhenn AM, Dunne AA, Werner JA. Complications after CO(2) laser surgery of laryngeal cancer in the elderly. Acta Otolaryngol. 2006;126(5):530-535.
doi pubmed - Lalami Y, de Castro G, Jr., Bernard-Marty C, Awada A. Management of head and neck cancer in elderly patients. Drugs Aging. 2009;26(7):571-583.
doi pubmed - Zabrodsky M, Calabrese L, Tosoni A, Ansarin M, Giugliano G, Bruschini R, Tradati N, et al. Major surgery in elderly head and neck cancer patients: immediate and long-term surgical results and complication rates. Surg Oncol. 2004;13(4):249-255.
doi pubmed - Clayman GL, Eicher SA, Sicard MW, Razmpa E, Goepfert H. Surgical outcomes in head and neck cancer patients 80 years of age and older. Head Neck. 1998;20(3):216-223.
doi - Eskander A, Irish J, Freeman J, Gullane P, Gilbert R, Groome PA, Hall SF, et al. Overview of major salivary gland cancer surgery in Ontario (2003-2010). J Otolaryngol Head Neck Surg. 2014;43:50.
doi pubmed - Wilson RT, Moore LE, Dosemeci M. Occupational exposures and salivary gland cancer mortality among African American and white workers in the United States. J Occup Environ Med. 2004;46(3):287-297.
doi pubmed - Carvalho AL. Racial/ethnic differences in head and neck cancer survival rates. Department of Public Health University of Washington; 2017.
- Suzuki I. Racial disparities in outcome among head and neck cancer patients in the United States: an analysis using SEER-Medicare linked database. Journal of Clinical Oncology. 2019;37(15):6051-6051.
doi - Goodwin WJ, Thomas GR, Parker DF, Joseph D, Levis S, Franzmann E, Anello C, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30(3):358-371.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


