| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 3, June 2022, pages 136-144
Long-Term Survival in Patients With Papillary Thyroid Cancer Who Did Not Undergo Prophylactic Central Lymph Node Dissection: A SEER-Based Study
Jun Long Songa, Ling Rui Lia, Zhi Liang Xua, Juan Juan Lia, Sheng Rong Suna, Chuang Chena, b
aDepartment of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China
bCorresponding Author: Chuang Chen, Department of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China
Manuscript submitted April 4, 2022, accepted May 21, 2022, published online June 2, 2022
Short title: pCLND and Survival
doi: https://doi.org/10.14740/wjon1483
| Abstract | ▴Top |
Background: The role of prophylactic central lymph node dissection (pCLND) for papillary thyroid cancer (PTC) remains contentious, and the impact of pCLND on long-term patient outcomes is unclear.
Methods: A retrospective analysis of data from the Surveillance, Epidemiology, and End Results (SEER) database was performed. Patients diagnosed with PTC who did not undergo pCLND between 2004 and 2015 were included in this study, and patients with pN0 PTC who underwent CLND were included as the control group. The researchers calculated the subdistribution hazard ratio (SHR) using the Fine-Gray model and the hazard ratio (HR) using the Cox proportional hazards regression to compare Thyroid cancer-specific survival (TCSS) and overall survival (OS) of the different groups.
Results: A total of 38,205 T1-2cN0 PTC patients without pCLND were eligible for the study entry, and 24,157 patients with T1-2pN0 PTC patients who had received CLND were included as the control group. The actuarial 10-year TCSS and OS rates of patients without pCLND were 99.53% and 92.77%, respectively. Patients without pCLND had similar TCSS compared with the control group after adjusting for age, sex, race, tumor stage, multifocality, thyroid surgery, and radiation (SHR = 1.35, 95% CI: 0.95 - 1.93). However, patients without pCLND had a significantly poorer OS than the control group (HR = 1.38, 95% CI: 1.26 - 1.51).
Conclusions: Patients without pCLND had similar TCSS compared with the control group after adjusting for confounders but had significantly poorer OS. Whether the OS disparities were attributed to pCLND or other factors still needs further study.
Keywords: Papillary thyroid cancer; Prophylactic central lymph node dissection; Survival; SEER database
| Introduction | ▴Top |
The incidence of thyroid cancer in China has increased significantly over the past two decades [1]. Papillary thyroid cancer (PTC) is the most common histologic subtype of thyroid cancer, accounting for 90% of new cases, and it has the best prognosis. Increased detection of subclinical thyroid cancers by increased utilization of ultrasonography and fine-needle biopsies may account for the rise in the incidence of thyroid cancer [2]. Surgery is the preferred treatment for PTC. Most patients with PTC will undergo surgical treatment consisting of thyroidectomy with or without lymph node dissection, as dictated by the extent of disease noted during preoperative evaluation and intraoperative inspection. For patients with suspicion of cervical lymph node metastases, a therapeutic compartment-orientated neck dissection of the involved lymph node basins should be included. However, for patients without any signs of cervical lymph node metastases, prophylactic central lymph node dissection (pCLND) is still under investigation [3-5].
The prevalence of lymph node metastases has been reported to be high in the central compartment (level VI). Clinically evident nodal metastases could be evaluated by clinical examination and preoperative ultrasound. However, only 20-30% of nodal metastases could be detected by clinical examination or preoperative ultrasound [6-8]. These methods have been shown to be unreliable in determining the presence of microscopic lymph node metastases. Lymph node metastases of level VI are more frequently occult and are not detectable preoperatively. Therefore, some cN0 PTC might have central lymph node metastases. Studies have reported that over 24% of lymph node metastases were detected in pCLND [9, 10]. Lymph node metastases have been reported to be associated with a higher risk of recurrence, and there are studies showing that their surgical removal improves survival rates [11-13]. The role of pCLND remains contentious. Many clinicians have suggested routine pCLND in PTC patients, particularly in China and Japan [14]. Routine pCLND may improve the accuracy of PTC nodal staging, reduce the burden of disease through excision of lymph node metastasis, and subsequently decrease the risk of loco-regional recurrence [15-17]. However, most PTC patients have a good prognosis, and mortality is low. When considering pCLND, the complication rates, such as recurrent laryngeal nerve injury and hypoparathyroidism associated with more extensive surgery that may affect the quality of life, should be taken into account [15, 18].
The risk factors for lymph node metastases include age at diagnosis, size of the primary tumor, and extrathyroidal extension [19-21]. In our clinical practice, patients with larger tumors and extrathyroidal extensions more frequently receive pCLND, while patients with low-risk tumors are less likely to receive it. It is generally accepted that pCLND does not change the overall prognosis but may affect recurrence rates. Many studies have evaluated the impact of pCLND on recurrence rates, but there are no convincing studies to evaluate the impact of pCLND on the survival of PTC. Therefore, we carried out this study to detect the impact of pCLND on the survival of PTC using data extracted from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database.
| Materials and Methods | ▴Top |
Data source and population
The SEER database is one of the most representative cancer-related databases and provides information on cancer incidence and survival derived from population-based cancer registries covering about one-third of the US population. For this study, SEER-18 data, which included 18 population-based cancer registries, were utilized. These data are available to the public with a signed data use agreement form (https://seer.cancer.gov/data/). Upon acceptance of the agreement, SEER*Stat software and data files were downloaded directly from the SEER website. Case lists were generated using SEER*Stat 8.3.9.2.
Data on thyroid cancer between 2004 and 2015 were obtained from the SEER database. SEER routinely collects information on procedures for removal, biopsy, or aspiration of regional lymph nodes performed during the initial workup or first course of therapy. The scope of regional lymph node surgery was used to determine whether the patients received lymph node dissection. If the scope of the regional lymph node surgery was unknown, the regional nodes examined by the pathologist were used to further determine the status of the lymph node dissection. Because most of the patients with T3-4 (AJCC, sixth edition) tumors would receive pCLND in clinical practice, in this study, we mainly evaluated the survival of those PTC patients with AJCC T1-2 disease and without pCLND. The eligibility criteria were as follows: 1) year of diagnosis from 2004 to 2015; 2) diagnosed with PTC; 3) staged with AJCC T1-2N0 disease; and 4) without lymph node removal. A control group consisting of PTC patients with lymph node removal was also established. The SEER database did not provide information on whether CLND was pCLND or therapeutic CLND. Therefore, those patients who received CLND and were staged with T1-2N0 disease were included as the control group. The eligibility criteria were as follows: 1) year of diagnosis from 2004 to 2015; 2) diagnosed with PTC; 3) staged with AJCC T1-2N0 disease; and 4) lymph node removal. Patients with no record of survival time were excluded.
The following demographic characteristics and clinical features for PTC were recorded: age at diagnosis, sex, race (white, black, or other), histological classification of thyroid cancer, AJCC tumor stage (T1, T2), multifocality (solitary, multifocal), thyroid surgery, regional lymph node surgery, and radiation. The extent of thyroid surgery was classified into lobectomy with isthmus or less (including lobectomy, isthmectomy, local excision, and others) and more than lobectomy with isthmus (including removal of a lobe and partial removal of the contralateral lobe, and subtotal or near total or total thyroidectomy). Radiotherapy included radioiodine therapy, beam radiation, and brachytherapy. Thyroid cancer-specific survival (TCSS) and overall survival (OS) were the primary outcomes of this study. Follow-up time was calculated from the date of initial diagnosis of thyroid cancer to death by any cause, lost to follow-up, or end of follow-up, whichever occurred first. Cause of death was defined according to the International Classification of Disease (ICD) codes and classified as attributable to the index cancer and other causes (https://seer.cancer.gov/causespecific/).
Statistical analysis
The demographic characteristics and clinical features of the patients in the different groups were compared using descriptive statistics. A Chi-square test was conducted to compare frequency distributions for categorical variables, and a two-tailed t-test was used to compare continuous variables, where appropriate. The Kaplan-Meier method was used to generate survival curves, and the log-rank test was performed to compare unadjusted TCSS and OS between the different groups. Because most of the PTC patients died from causes other than thyroid, to take enough account of the competing risks, a univariate and multivariate Fine and Gray competing-risk regression model, a semi-proportional model that provides estimates of the cumulative incidence function of each event as a log subdistribution hazard ratio (SHR), was built to analyze TCSS. Univariate and multivariate analyses were carried out using the Cox proportional hazards regression to compare the OS between different groups. In the multivariate analyses, the researchers adjusted for confounders, including age, sex, race, T stage, multifocality, thyroid surgery, and radiation. Subgroup analysis for TCSS and OS was performed according to age group, sex, and T stage. All statistical calculations were performed using Stata version 15.1 (StataCorp, College Station, TX). All P values were two-tailed. The significance level was set at P < 0.05. The work has been reported in line with the STROCSS criteria [22].
Ethical compliance with human/animal study
The SEER program collects data from population-based cancer registries with anonymous information. The SEER is a publicly available database and data extracted from SEER was deemed “non-human study” by the North Shore LIJ IRB committee, thus no ethical approval is required.
| Results | ▴Top |
Baseline characteristics
In total, 38,205 PTC patients without pCLND were eligible for the study between 2004 and 2015, and 24,157 patients with T1-2N0 PTC patients who had received CLND were included as the control group. Table 1 shows the baseline characteristics of the included patients in the different groups. The patients without pCLND were much older at the diagnosis of thyroid cancer than the control group (mean age: 50.02 vs. 46.98; P < 0.001). A higher proportion of females received CLND than males (P < 0.001). White patients were more likely to receive CLND than other races (P < 0.001). More patients with multifocal disease received CLND (P < 0.001). In the CLND group, more patients received larger thyroid resection scope (P < 0.01), and more patients with CLND received radiation (P < 0.001). Regarding the tumor stage, no significant difference was identified between patients with and without CLND (P = 0.267).
 Click to view | Table 1. Baseline Characteristics of Thyroid Cancer Patients According to Prophylactic Central Lymph Node Dissection |
Survival analysis
After a median of 79 months of follow-up, a total of 2,545 (4.08%) patients died at the end of the study follow-up. In the group of patients without pCLND, 1,903 (4.98%) had died, whereas 642 (2.66%) patients in the control group had died. Among the 2,545 patients who died, only 166 (6.52%) patients died from thyroid cancer, and 2,527 (93.48%) patients died due to other causes. The actuarial 10-year TCSS rate of patients without pCLND was 99.53% (95% confidence interval (CI): 99.42% - 99.62%), whereas it was 99.68% (95% CI: 99.55% - 99.78%) for patients with CLND. The actuarial 10-year OS of patients without pCLND was 92.77% (95% CI: 92.41% - 93.13%), whereas it was 95.73% (95% CI: 95.33% - 96.09%) for patients with CLND.
Kaplan-Meier plots were generated to compare the TCSS and OS of the different groups, and the results are shown in Figure 1. In general, patients without pCLND were more likely to experience all-cause mortality and thyroid cancer-specific mortality than the control group. A univariate and multivariate Fine and Gray competing-risk regression model was used to analyze the TCSS. As shown in Table 2, in the univariate model, patients without pCLND had significantly poorer TCSS than the control group (patients without pCLND vs. the control group: SHR = 1.58, 95% CI: 1.12 - 2.23). In further multivariate analyses, patients without pCLND had similar TCSS to patients with CLND after adjusting for confounders (patients without pCLND vs. the control group: SHR = 1.35, 95% CI: 0.95 - 1.93). We further analyzed the OS differences between the different groups. Table 3 summarizes the crude and adjusted HRs (95% CI) for OS between patients without pCLND and patients with CLND. In the univariate analysis, patients without pCLND had significantly poorer OS than the control group (patients without pCLND vs. the control group: HR = 1.72, 95% CI: 1.57 - 1.88). After adjusting for confounders, patients without pCLND still had poorer OS than patients with CLND (patients without pCLND vs. the control group: HR = 1.38, 95% CI: 1.26 - 1.51).
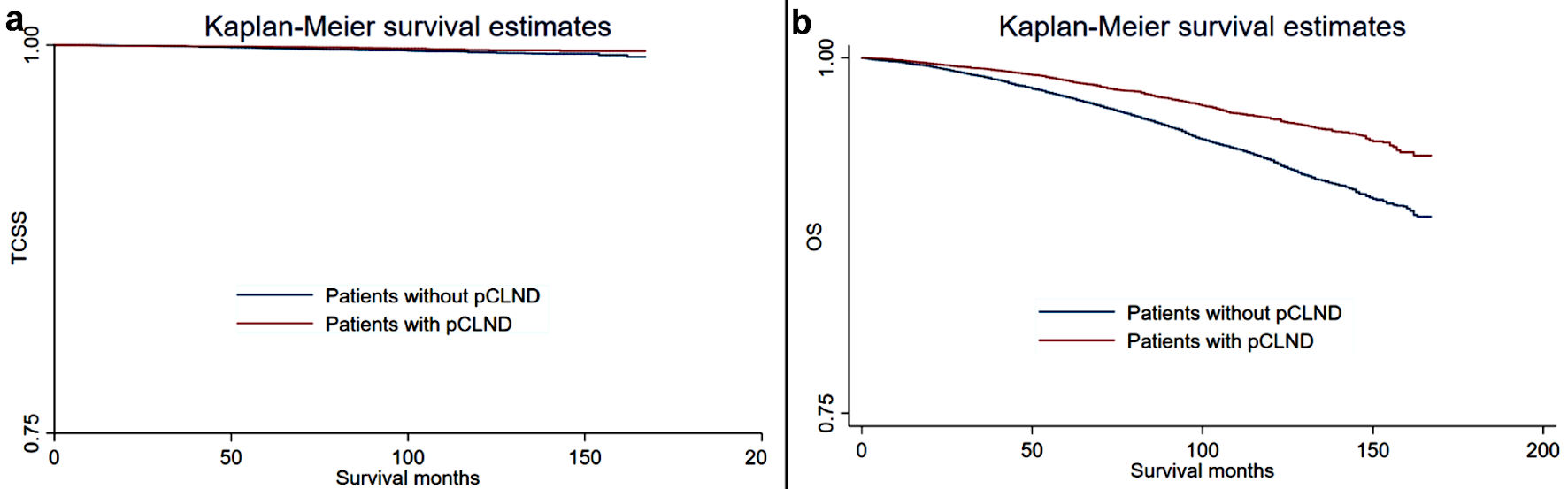 Click for large image | Figure 1. Kaplan-Meier curves for thyroid cancer-specific survival (TCSS) and overall survival (OS) in patients with and without prophylactic central lymph node dissection (pCLND). (a) Thyroid cancer-specific survival. (b) Overall survival. |
 Click to view | Table 2. Univariate Variate and Multivariate Fine-Gray Competing Risk Model for Thyroid Cancer Specific Survival |
 Click to view | Table 3. Univariate and Multivariate Cox Proportional Hazards Regression for Overall Survival |
The researchers also carried out further stratified analyses of different age groups, sex, and different T stages for TCSS and OS. As shown in Table 4, patients without pCLND had similar TCSS compared with the control group in different subgroups, adjusted for confounders. Patients without pCLND had poor OS in all subgroups after adjusting for confounders.
 Click to view | Table 4. Assessing the Survival of Patients Without pCLND Using Multivariate Fine-Gray Model for TCSS and Cox Proportional Hazards Regression Model for OS in Different Subgroups |
| Discussion | ▴Top |
To our knowledge, this is the first study that was performed with the aim of exploring the long-term survival of PTC patients without pCLND. Due to the excellent outcomes, low recurrence rate, and mortality rate of PTC, it is not feasible to conduct a large prospective randomized clinical study to clarify this issue. We carried out this population-based, large-scale retrospective study to further detect the survival rate of PTC patients without pCLND. In our study, patients without pCLND were much older. The old patients were more likely to have comorbidities and the surgeons tended to perform less extensive surgery if they had no obvious central lymph node involvement. And patients without pCLND were also less likely to undergo total thyroidectomy in our study. Furthermore, more T1 and unifocal tumors were observed in patients without pCLND. These tumors had a low risk of metastasis to lymph nodes. In further survival analysis, patients without pCLND had similar TCSS compared with the control group after adjusting for age, sex, race, tumor stage, multifocality, thyroid surgery, and radiation (SHR = 1.35, 95% CI: 0.95 - 1.93), but they had significantly poorer OS (HR = 1.38, 95% CI: 1.26 - 1.51). In the stratified analyses, the same trend was observed in all subgroups.
One of the most controversial issues in the therapy of PTC is pCLND. The performance of pCLND in clinically node-negative PTC varied a lot in different thyroid centers. Occult lymph node metastasis is common in PTC, especially in the central compartment. Theoretically, pCLND could remove occult metastasis and decrease the recurrence rate. Another potential benefit of pCLND has been to more accurately stage patients, which may affect radioactive iodine (RAI) treatment [23]. Some studies have demonstrated improved outcomes with nodal dissection. Medas et al found that occult lymph node metastases were found in 25.3% of patients in the pCLND group, and occult lymph node metastases resulted as a significant negative prognostic factor for recurrence [10]. In a meta-analysis carried out by Zhao et al, patients with pCLND had a significantly reduced risk of locoregional recurrence [17]. However, many other studies showed that pCLND did not improve tumor control and had a higher risk of developing complications, such as hypocalcemia [24-26]. A prospective randomized controlled study by Viola et al concluded that pCLND did not reduce the central neck recurrence rate, with the incidence of permanent hypoparathyroidism increasing significantly [24]. Due to the excellent outcomes of PTC, most studies have not demonstrated significant differences in TCSS [27-29]. In our analysis, we demonstrated a similar long TCSS period between patients without pCLND and the control group. The 10-year TCSS of patients without pCLND was 99.47%, which is similar to the results of previous studies [28, 29].
Interestingly, in our study, we demonstrated poor OS in patients without pCLND, which was not reported in previous studies. Several mechanisms may explain the poor OS in patients without pCLND. One possible explanation is that pCLND may be a potential protective factor for OS because patients without pCLND have a higher risk of developing recurrence and metastasis [10]. Patients may also die from complications secondary to recurrence or metastasis, which may be recorded as death for other reasons. However, a more reasonable explanation is that this survival disparity was caused by baseline comorbidities with the diagnosis of PTC. Comorbidities can increase the risk of non-thyroid cancer mortality [30]. Patients with comorbidities, such as cardiovascular disease and other malignancies, would be less likely to undergo pCLND if there were no clinically positive lymph nodes. Actually, in our clinical work, we tend to perform less extensive surgery on patients with comorbidities, such as heart disease or cancer. In this study, patients with pCLND were much older than the control group (mean age: 50.02 vs. 46.98), which meant that patients without pCLND had a higher probability of having comorbidities. However, as baseline comorbidities were not recorded in the SEER database, we could not conduct a further analysis. However, in this study, more patients without pCLND died from other causes, such as cardiovascular disease and other cancers, rather than thyroid cancer. Studies have also reported that patients with comorbidities were more likely to undergo less extensive surgery [31-33]. However, most of the present studies focused on recurrence-free survival or TCSS. Few studies have reported OS disparities between patients with and without pCLND. Further studies are needed to clarify the association between pCLND and the OS of PTC.
The notable strengths of this study include the large patient cohort and long-term active follow-up of survival. However, there were also several limitations that need to be noted. First, SEER provides no information about thyroid-stimulating hormone (TSH) suppression therapy. The potential adverse consequences of TSH suppression may develop and cause increased cardiovascular risks [34]. Second, there was a variety of information that was not routinely collected in the SEER, such as body mass index and medical comorbidities. Comorbidities are known to increase the risk for non-cancer mortality that could affect the pCLND-associated survival disparities. Finally, SEER only included whether lymph node dissection was performed, but information about pCLND was not recorded. Therefore, we could not make a direct comparison between patients with pCLND and patients without pCLND. Instead, those patients with N0 PTC who underwent CLND were chosen as the control group.
Conclusions
In conclusion, in this large population-based analysis, patients without pCLND had excellent long-term TCSS. Patients without pCLND had similar TCSS compared with the control group after adjusting for confounders but had significantly poorer OS. Whether the OS disparities were attributed to pCLND or other factors still needs further study.
Acknowledgments
The authors gratefully thank the SEER program for open access to their database.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patients by the SEER program.
Author Contributions
Jun Long Song: conceptualization, data curation, formal analysis, supervision, and writing - original draft. Ling Rui Li: conceptualization, formal analysis, writing - original draft. Zhi Liang Xu: conceptualization, writing - review and editing. Juan Juan Li: investigation, methodology, and supervision. Sheng Rong Sun: resources, writing - review and editing. Chuang Chen: conceptualization, supervision, formal analysis, writing - review and editing.
Data Availability
Data from the Surveillance, Epidemiology, and End Results (SEER) database are available on reasonable request.
| References | ▴Top |
- Wang J, Yu F, Shang Y, Ping Z, Liu L. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine. 2020;68(1):163-173.
doi pubmed - Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol. 2018;14(11):670-683.
doi pubmed - Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19(7):683-689.
doi pubmed - Gyorki DE, Untch B, Tuttle RM, Shaha AR. Prophylactic central neck dissection in differentiated thyroid cancer: an assessment of the evidence. Ann Surg Oncol. 2013;20(7):2285-2289.
doi pubmed - Glover AR, Gundara JS, Norlen O, Lee JC, Sidhu SB. The pros and cons of prophylactic central neck dissection in papillary thyroid carcinoma. Gland Surg. 2013;2(4):196-205.
- Moreno MA, Edeiken-Monroe BS, Siegel ER, Sherman SI, Clayman GL. In papillary thyroid cancer, preoperative central neck ultrasound detects only macroscopic surgical disease, but negative findings predict excellent long-term regional control and survival. Thyroid. 2012;22(4):347-355.
doi pubmed - Mazzaferri EL. Long-term outcome of patients with differentiated thyroid carcinoma: effect of therapy. Endocr Pract. 2000;6(6):469-476.
doi pubmed - Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: Diagnosis of central and lateral compartment nodal metastases. Eur J Radiol. 2019;112:14-21.
doi pubmed - Hughes DT, Rosen JE, Evans DB, Grubbs E, Wang TS, Solorzano CC. Prophylactic central compartment neck dissection in papillary thyroid cancer and effect on locoregional recurrence. Ann Surg Oncol. 2018;25(9):2526-2534.
doi pubmed - Medas F, Canu GL, Cappellacci F, Anedda G, Conzo G, Erdas E, Calo PG. Prophylactic central lymph node dissection improves disease-free survival in patients with intermediate and high risk differentiated thyroid carcinoma: a retrospective analysis on 399 patients. Cancers (Basel). 2020;12(6):1658.
doi pubmed - Amit M, Tam S, Boonsripitayanon M, Cabanillas ME, Busaidy NL, Grubbs EG, Lai SY, et al. Association of lymph node density with survival of patients with papillary thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2018;144(2):108-114.
doi pubmed - Smith BD, Oyekunle TO, Thomas SM, Puscas L, Rocke DJ. Association of lymph node ratio with overall survival in patients with metastatic papillary thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2020;146(10):962-964.
doi pubmed - Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. 2016;34(36):4415-4420.
doi pubmed - Takami H, Ito Y, Okamoto T, Onoda N, Noguchi H, Yoshida A. Revisiting the guidelines issued by the Japanese Society of Thyroid Surgeons and Japan Association of Endocrine Surgeons: a gradual move towards consensus between Japanese and western practice in the management of thyroid carcinoma. World J Surg. 2014;38(8):2002-2010.
doi pubmed - Chen L, Wu YH, Lee CH, Chen HA, Loh EW, Tam KW. Prophylactic central neck dissection for papillary thyroid carcinoma with clinically uninvolved central neck lymph nodes: a systematic review and meta-analysis. World J Surg. 2018;42(9):2846-2857.
doi pubmed - Lang BH, Ng SH, Lau LL, Cowling BJ, Wong KP, Wan KY. A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid. 2013;23(9):1087-1098.
doi pubmed - Zhao W, You L, Hou X, Chen S, Ren X, Chen G, Zhao Y. The effect of prophylactic central neck dissection on locoregional recurrence in papillary thyroid cancer after total thyroidectomy: a systematic review and meta-analysis: pCND for the locoregional recurrence of papillary thyroid cancer. Ann Surg Oncol. 2017;24(8):2189-2198.
doi pubmed - Dobrinja C, Troian M, Cipolat Mis T, Rebez G, Bernardi S, Fabris B, Piscopello L, et al. Rationality in prophylactic central neck dissection in clinically node-negative (cN0) papillary thyroid carcinoma: Is there anything more to say? A decade experience in a single-center. Int J Surg. 2017;41(Suppl 1):S40-S47.
doi pubmed - Liu C, Xiao C, Chen J, Li X, Feng Z, Gao Q, Liu Z. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer. 2019;19(1):622.
doi pubmed - Yan B, Hou Y, Chen D, He J, Jiang Y. Risk factors for contralateral central lymph node metastasis in unilateral cN0 papillary thyroid carcinoma: A meta-analysis. Int J Surg. 2018;59:90-98.
doi pubmed - Mao J, Zhang Q, Zhang H, Zheng K, Wang R, Wang G. Risk factors for lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:265.
doi pubmed - Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G, STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156-165.
doi pubmed - Shirley LA, Jones NB, Phay JE. The role of central neck lymph node dissection in the management of papillary thyroid cancer. Front Oncol. 2017;7:122.
doi pubmed - Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, Seccia V, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab. 2015;100(4):1316-1324.
doi pubmed - De Napoli L, Matrone A, Favilla K, Piaggi P, Galleri D, Ambrosini CE, Aghababyan A, et al. Role of prophylactic central compartment lymph node dissection on the outcome of patients with papillary thyroid carcinoma and synchronous ipsilateral cervical lymph node metastases. Endocr Pract. 2020;26(8):807-817.
doi pubmed - Dismukes J, Fazendin J, Obiarinze R, Marquez GCH, Ramonell KM, Buczek E, Lindeman B, et al. Prophylactic central neck dissection in papillary thyroid carcinoma: all risks, no reward. J Surg Res. 2021;264:230-235.
doi pubmed - Jo YJ, Choi HR, Park SH, Jeong YJ. Extent of thyroid surgery for clinically node-negative papillary thyroid carcinoma with confirmed nodal metastases after prophylactic central neck dissection: a 15-year experience in a single center. Ann Surg Treat Res. 2020;99(4):197-204.
doi pubmed - Huang H, Wu L, Liu W, Liu J, Liu Y, Xu Z. Long-term outcomes of patients with papillary thyroid cancer who did not undergo prophylactic central neck dissection. J Cancer Res Ther. 2020;16(5):1077-1081.
doi pubmed - Nixon IJ, Ganly I, Patel SG, Morris LG, Palmer FL, Thomas D, Tuttle RM, et al. Observation of clinically negative central compartment lymph nodes in papillary thyroid carcinoma. Surgery. 2013;154(6):1166-1172; discussion 1172-1163.
doi pubmed - Du B, Wang F, Wu L, Wang Z, Zhang D, Huang Z, Gao L, et al. Cause-specific mortality after diagnosis of thyroid cancer: a large population-based study. Endocrine. 2021;72(1):179-189.
doi pubmed - Suman P, Razdan SN, Wang CE, Tulchinsky M, Ahmed L, Prinz RA, Winchester DJ. Thyroid lobectomy for T1b-T2 papillary thyroid cancer with high-risk features. J Am Coll Surg. 2020;230(1):136-144.
doi pubmed - Zambeli-Ljepovic A, Wang F, Dinan MA, Hyslop T, Stang MT, Roman SA, Sosa JA, et al. Extent of surgery for low-risk thyroid cancer in the elderly: Equipoise in survival but not in short-term outcomes. Surgery. 2019;166(5):895-900.
doi pubmed - Lohia S, Gupta P, Curry M, Morris LGT, Roman BR. Life expectancy and treatment patterns in elderly patients with low-risk papillary thyroid cancer: a population-based analysis. Endocr Pract. 2021;27(3):228-235.
doi pubmed - Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010;20(2):135-146.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


