| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 4, August 2022, pages 195-204
Pathological Responses of the Primary Tumor and Locoregional Lymph Nodes After Neoadjuvant Immunochemotherapy in Esophageal Squamous Cell Cancer
Shu Jie Huanga, b, i, Dan Tiana, i, Si Chao Wanga, i, Rui Jie Zengb, c, Yue Jiao Dongb, d, Liang Li Hongb, d, Han Sheng Wub, e, Fang Ping Xuf, Dong Kun Zhanga, Liang Xiea, Hai Yu Zhoua, Ji Ming Tanga, Xiao Song Bena, Gang Chena, Ri Xin Chena, g, Yong Tanga, j, Gui Bin Qiaoa, b, h, j
aDepartment of Thoracic Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China
bShantou University Medical College, Shantou 515041, China
cDepartment of Gastroenterology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
dDepartment of Pathology, The First Affiliated Hospital of Shantou University Medical College, Shantou, China
eDepartment of Thoracic Surgery, The First Affiliated Hospital of Shantou University Medical College, Shantou, China
fDepartment of Pathology and Laboratory Medicine, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China
gResearch Center of Medical Sciences, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China
hThe Second School of Clinical Medicine, Southern Medical University, Guangzhou 510515, China
iThey contributed equally to this work.
jCorresponding Author: Gui Bin Qiao, Department of Thoracic Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China; Yong Tang, Department of Thoracic Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China
Manuscript submitted May 6, 2022, accepted June 23, 2022, published online August 23, 2022
Short title: Pathological Responses in Esophageal Cancer
doi: https://doi.org/10.14740/wjon1489
| Abstract | ▴Top |
Background: The current study attempted to describe the specific patterns of pathological tumor response and locoregional node metastases from surgically resected esophageal squamous cell carcinoma after neoadjuvant immunochemotherapy (NAIC), as well as to explore the association between clinicopathological characteristics and such oncological patterns.
Methods: Fifty-one patients with cT3 or deeper esophageal squamous cell cancer underwent subtotal esophagectomy after NAIC. The NAIC regimen included intravenous administration of platinum-based and docetaxel- and taxane-based chemotherapeutics along with a 200 mg fixed dose of one programmed death 1 (PD-1) inhibitor, given every 3 weeks. We divided patients into tumor/nodal good-responders and poor-responders based on the pathological observation of the tumor or nodal responses. We also examined the association between clinicopathological factors and tumor/nodal responses. Further, significant baseline predictors for tumor and nodal good-responders were identified using multivariate binary logistic regression.
Results: Of the 51 patients, 68.6% achieved marked primary tumor response. Notably, 21.6% of patients achieved complete pathological response. Significant differences in treatment cycles between tumor good-responders and tumor poor-responders (P = 0.019) were observed. For locoregional nodal responses, only 33.3% of patients achieved down-staged nodal disease. Of the investigated variables, neoadjuvant cycles (odds ratio (OR): 5.271, 95% confidence interval (CI): 1.278 - 21.740, P = 0.022) and pretreatment platelets (OR: 0.979, 95% CI: 0.962 - 0.996, P = 0.017) were identified as independent predictors for good tumor and nodal responses.
Conclusions: We conclusively noted that most patients receiving NAIC were tumor good-responders, whereas only one-third of patients were nodal good-responders. Furthermore, we identified that treatment cycle number and baseline platelet counts were independent predictors of combined tumor and nodal responses.
Keywords: Esophageal squamous cell carcinoma; Neoadjuvant immunochemotherapy; Prediction model; Real-world
| Introduction | ▴Top |
Esophageal cancer (EC) has an incidence exceeding 0.6 million cases and is the sixth leading cause of death globally [1]. Despite advances in multimodal treatments, the overall prognosis for locally advanced EC is poor [2]. In Eastern Asia, esophageal squamous cell carcinoma (ESCC) is the dominant histological type of EC [3]. The rapidly fatal course of ESCC results in a low 5-year overall survival [4]. Neoadjuvant chemoradiotherapy has been regarded as the standard-of-care for locally advanced ESCC [5]. Since the JCOG9907 trial, neoadjuvant chemotherapy has been regarded as a standard treatment alternative for locally advanced EC due to its superiority over adjuvant chemotherapy [6].
Clinical and histopathological responses of target tumor lesion and local-distant micrometastatic sites (e.g., regional lymph node metastasis or distant micrometastases) are considered to be independent prognosticators for EC [7-9]. The research on residual tumor patterns and locoregional lymph node metastases of the resected specimen was found to be compelling. A few studies have addressed this issue via multiple approaches [8-12]. Recent studies on patients with EC showed satisfying feasibility, efficacy, and safety of a novel treatment strategy - the neoadjuvant immunotherapy combined with chemotherapy (neoadjuvant immunochemotherapy (NAIC)) [13-18]. However, little is known about the pathological tumor response and residual nodal metastasis in patients with ESCC after NAIC.
This study aims to first describe the specific patterns of pathological tumor response and locoregional node metastases from surgically resected specimens after NAIC, and then to explore the association between clinicopathological characteristics and oncological patterns.
| Materials and Methods | ▴Top |
Patients
We conducted a retrospective study including ESCC patients who underwent NAIC followed by surgery at the Department of Thoracic Surgery, Guangdong Provincial People’s Hospital from March 2019 to January 2021. The last date of follow-up in the study was October 1, 2021. The inclusion criteria were as follows: 1) pathologically confirmed ESCC; 2) cT3 or deeper clinical T stage; 3) underwent surgical resection after completion of neoadjuvant therapy; 4) complete and retrievable clinical records. Patients receiving upfront surgery or diagnosed with any M1 disease were excluded. The schematic diagram of the patient selection process is presented in Figure 1.
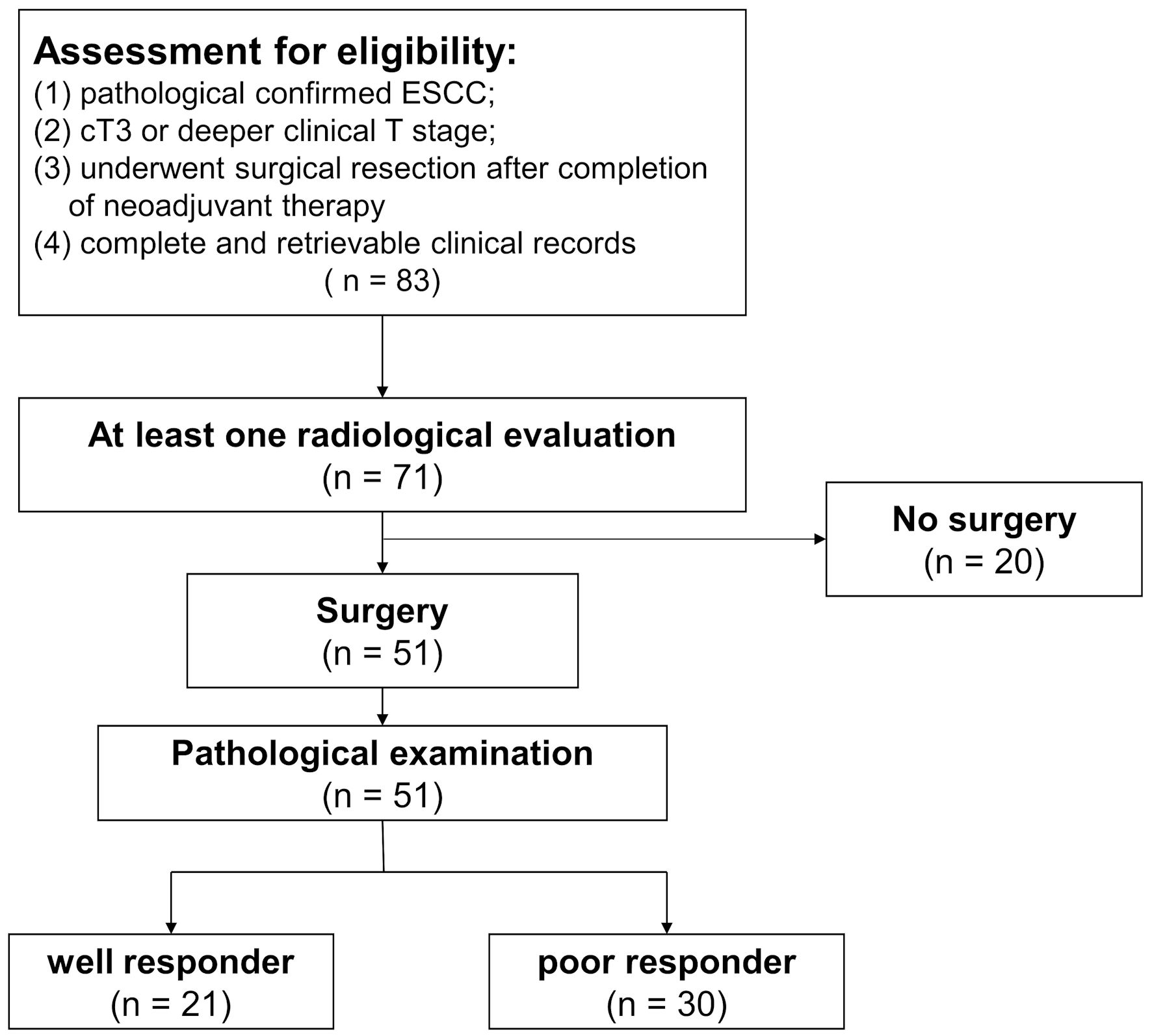 Click for large image | Figure 1. Flowchart of patient eligibility for inclusion. Of the 83 patients receiving neoadjuvant immunochemotherapy, 71 patients were evaluated at least once via radiological imaging. Eventually, a total of 51 patients underwent surgery. Based on the response of primary tumor and lymph nodes, patients were categorized into different groups. ESCC: esophageal squamous cell carcinoma. |
Preoperative workup
Before initiating NAIC, the diagnosis and clinical stage of the enrolled patients were confirmed using endoscopy-guided biopsy, contrast-enhanced computed tomography (CT), and positron emission tomography (PET). Immunotherapy based on the standard neoadjuvant chemotherapy were recommended for patients with ESCC who fit the inclusion criteria. All enrolled participants were fully informed and provided written consent. The NAIC regimen included intravenous administration of platinum-based and docetaxel-/taxane-based chemotherapeutics plus one kind of one programmed death 1 (PD-1) inhibitor, every 3 weeks intravenously. Administered PD-1 inhibitors included camrelizumab, nivolumab, pembrolizumab, sintilimab, and tislelizumab. After the completion of at least two cycles of NAIC, physical examination, routine laboratory tests, and contrast-enhanced thoracoabdominal CT or PET-CT were performed for disease evaluation. Radiological responses were recorded according to RECIST version 1.1 [19]. After a thorough evaluation of target and non-target lesions, the overall responses were denoted by complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) after a thorough evaluation of both target and non-target lesions. Patients with marked relief of cancer-related clinical symptoms and a radiological CR/PR disease were considered surgical candidates. Moreover, those with radiologically confirmed PD were deemed unfit for surgery.
Surgery
Subtotal esophagectomy with two-field lymphadenectomies were performed on medically fit patients. Our standard surgical procedure included transthoracic esophagectomy, reconstruction of the gastric tube, and cervical anastomosis between the esophageal and gastric stump.
Pathological diagnosis
The resected esophagus and lymph nodes were processed for further evaluation with a standardized protocol at our pathology department for further evaluation. Longitudinal sections of the resected specimens were embedded after formalin fixation. The specimens were then stained using hematoxylin and eosin (H&E) staining for microscopic examination and independently evaluated by two pathologists. Maximal tumor diameter, lymphovascular invasion, perineural invasion, R0 resection and lymph node positivity were assessed and reported. For study purposes, esophageal walls were divided into four layers: mucosa, submucosa, muscle and adventitia. Tumor regression grade (TRG) was evaluated per the Becker system, which is a four-tier scoring system estimating the percentage of residual tumor in relation to the macroscopically identifiable tumor bed [20]. These four grades include TRG1a: no residual tumor; TRG1b: < 10% residual tumor; TRG2: 10-50% residual tumor; TRG3: > 50% residual tumor cells with or without the signs of NAIC. According to the grade of primary tumor regression, we categorized patients into tumor good-responders (T-GRs) and tumor poor-responders (T-PRs). T-GRs were defined as patients who had TRG1a or TRG1b disease, whereas T-PR were patients who had TRG2 or TRG3 disease. Meanwhile, according to the grade of regional lymph node metastases, we categorized patients into node good-responders (N-GRs), node poor-responders (N-PRs) and node no-responders (N-NRs). N-GRs were defined as patients whose lymph node status were down-staged after neoadjuvant therapy; N-PRs were those who had stable or progressed N stage; N-NRs were defined as patients who had no signs of positive lymph node (cN0 to pN0) from beginning to end. We further classified patients who were both T-GRs and N-GRs/N-NRs into the TN-GRs group (tumor and nodal good-responders), whereas the rest of the patients were denoted as TN-PRs (tumor and nodal poor-responders). Moreover, we also adopted pathological complete response (pCR) and tumor-associated overall survival (OS). The pCR was defined as the absence of invasive/in situ cancer in the primary cancer site, and OS was defined as the time from diagnosis to tumor-associated death or last follow-up.
Ethical approval
The present study was approved by the ethics committee of Guangdong Provincial People’s Hospital (No. GDREC2020195H(R1)). All the procedures in this study were performed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national). This study was in compliance with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients.
Statistical analysis
Descriptive data were reported as mean ± standard deviation, median (interquartile range (IQR)), or percentage. Comparisons of continuous variables were performed using Student’s t-test and Wilcoxon-rank sum test. Categorical clinicopathological factors were compared using the Chi-square test or Fisher’s exact test. P < 0.05 was considered statistically significant. Multivariate binary logistic regression models were built to identify independent pretreatment factors for therapeutic outcomes. These models were built via a three-step approach [21]. Forward stepwise approach was adopted. Potential confounders such as sex, height, history of smoking, history of drinking, and family oncological history were included in the models to rule out their effects. All statistical analyses were performed using the software Statistical Package for Social Science (SPSS) version 26 for Windows (SPSS, Inc, Chicago, Illinois) and R 4.0.0 (R Core Team 2020) [22]. High-quality figures were generated using the R packages.
| Results | ▴Top |
Patient characteristics
Of the 83 enrolled patients, 71 completed the planned treatment course. Eventually, 51 (71.8% in 71) were considered suitable for surgery and underwent esophagectomy. The male-to-female ratio was 4:1, and the median age of the cohort was 60 years (IQR: 54 - 65). Most primary tumors were located in the middle (45.1%) or lower portion (41.2%) of the thoracic esophagus. Overall, 45.1% of ESCC patients received ≥ 2 cycles of NAIC. The detailed clinicopathological information is presented in Table 1.
 Click to view | Table 1. Clinicopathological Characteristics of Esophageal Squamous Cell Cancer Patients |
Pathological response of primary tumors and association with clinicopathological factors
The pathological tumor response pattern is presented in Figure 2a. Tumor invasion depths among the neoadjuvant recipients were at least T3 before initiation of treatment. Of the selected surgical candidates, tumor invasion depths were limited to the muscular layer in 68.6% of patients. The frequently involved residual tumor sites were identified in the mucosa and submucosa. Among the cT3 group, 45.2% of patients (n = 14) achieved TRG1a/1b; while 65% (n = 20) of patients in cT4 group reached TRG1a/1b. Notably, 11 patients (21.6%) had complete pathological response disease (pCR), while nine patients had carcinoma in situ disease. Less than 10% residual tumor was observed in 52.9% of the resected specimens (n = 27).
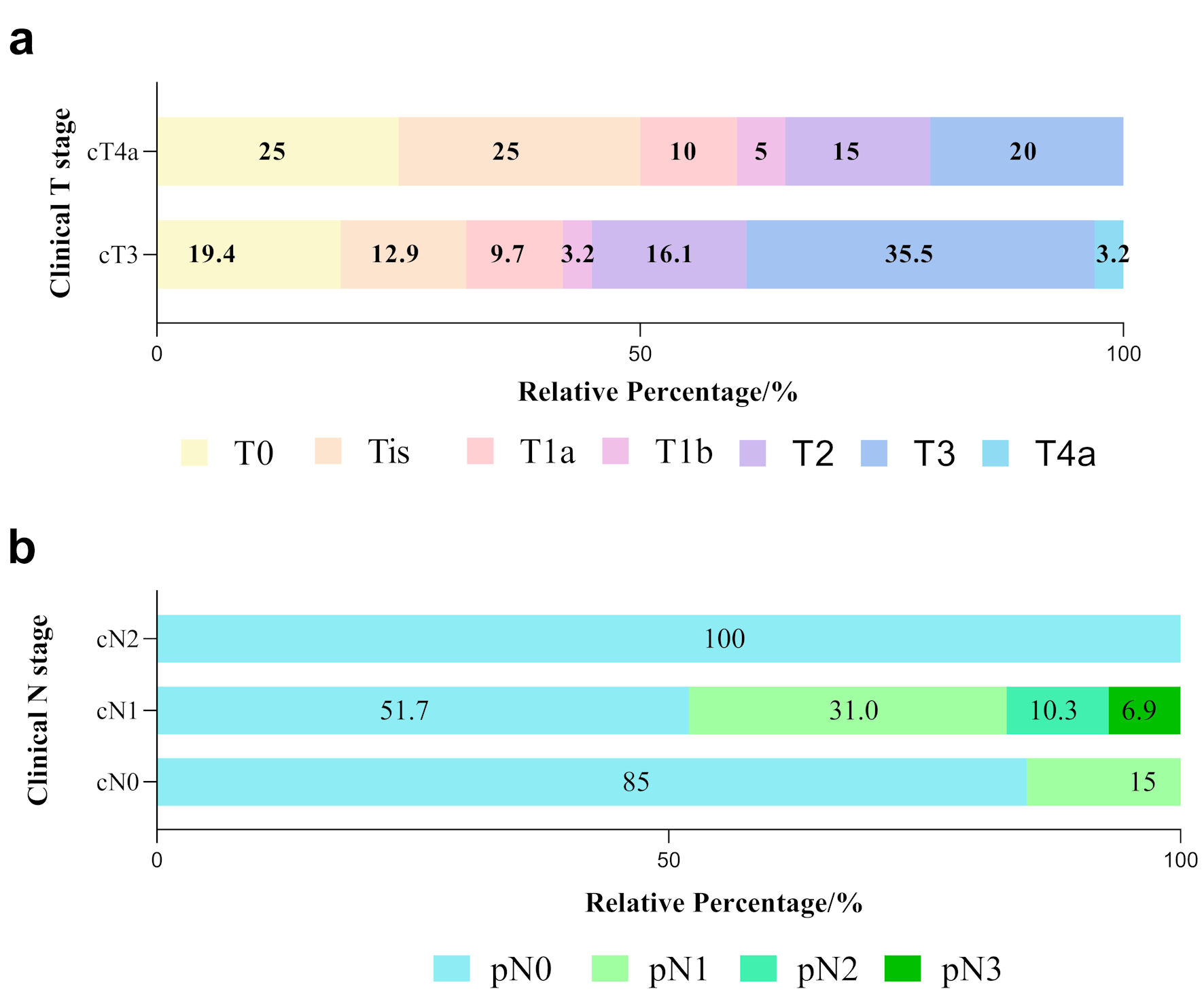 Click for large image | Figure 2. Tumor and nodal responses in ESCC patients with various clinical stages. (a) Differences in pathological T stage between cT3 and cT4a patients. Various color blocks represent depths of tumor invasion. (b) Differences in pathological lymph node response (represented by different color blocks) among cN0, cN1 and cN2 groups after neoadjuvant immunochemotherapy. ESCC: esophageal squamous cell carcinoma. |
We compared the clinicopathological factors between T-GRs and T-PRs. Significant differences were observed in age (P = 0.049), pretreatment neutrophil-to-lymphocyte (P = 0.006), pretreatment neutrophil ratio (P = 0.003), pretreatment lymphocyte ratio (P = 0.009), pretreatment neutrophil count (P = 0.036), neoadjuvant cycles (P = 0.017), pCR (P = 0.001), post-treatment primary tumor SUVmax (P = 0.042), lymphovascular invasion (P = 0.016), perineural invasion (P = 0.016), and ypTNM (P < 0.001). Notably, the proportion of T-GRs was significant higher in the group that received three cycles of NAIC than those with only two cycles (76.2% in three cycles vs. 35.7% in two cycles, P = 0.017 (< 0.05)). All of the T-GRs had both negative lymphovascular and perineural invasion, compared to 18.2% positive findings (n = 4) in T-PRs (P = 0.016). No significant association between immunohistochemical markers and T-GRs/PRs were observed.
Neoadjuvant locoregional lymph node metastases and their association with other clinicopathological factors
The pattern of locoregional lymph nodes metastases after NAIC was depicted (Fig. 2b). Patients with clinical N stage of N0-2 were enrolled. Of the 20 cN0 patients at pretreatment phase, three (15%) had pN1 disease after surgery. Notably, 15 out of 29 (51.7%) cN1 patients achieve pN0 status. However, five patients (17.2%) experienced more lymph node metastases. In contrast, two patients with cN2 before NAIC were free from node metastases. Only 29.4% of patients achieved down-staged N disease.
We compared the clinicopathological factors between N-GRs and N-PRs. Significant differences were observed in the pretreatment neutrophil-to-lymphocyte ratio (P = 0.006), pretreatment neutrophil ratio (P = 0.003), pretreatment lymphocyte ratio (P = 0.012), pretreatment neutrophil count (P = 0.036), pretreatment erythrocyte count (P = 0.025), posttreatment primary lesion SUVmax (P = 0.001), lymphovascular invasion (P = 0.026), perineural invasion (p = 0.029), pCR (P = 0.034), R0 (P = 0.009), and ypTNM (P < 0.001). Notably, the proportion of N-PRs was significantly higher in those with negative lymphovascular invasion (74.4% N-PRs vs. 25.6% N-GRs). Moreover, a higher proportion of R0 rate was higher in N-GRs (61.5%) compared to that in N-PRs (38.5%) (P = 0.009).
Combined primary tumor and residual lymph node response
To further explore the pretreatment clinicopathological predictors of T-responders and N-responders, we performed binary logistic regression analyses. Univariate analyses revealed that age (odds ratio (OR): 4.0, 95% confidence interval (CI): 1.225 - 13.056, P = 0.022), neoadjuvant treatment cycles (OR: 4.875, 95% CI: 1.428 - 16.641, P = 0.011), pretreatment neutrophil-to-lymphocyte ratio (OR: 1.433, 95% CI: 0.929 - 2.210, P = 0.039) were significantly associated with combined tumor and nodal responses (Table 2). After adjusted for potential cofounders, further multivariate regression analyses identified neoadjuvant cycles (OR: 5.271, 95% CI: 1.278 - 21.740, P = 0.022) and pretreatment platelets (OR: 0.979, 95% CI: 0.962 - 0.996, P = 0.017) as independent predictors for good tumor and nodal responses (Fig. 3).
 Click to view | Table 2. Univariate and Multivariate Binary Logistic Regression of Pretreatment Clinicopathological Factors for Prediction of Tumor and Nodal Responses |
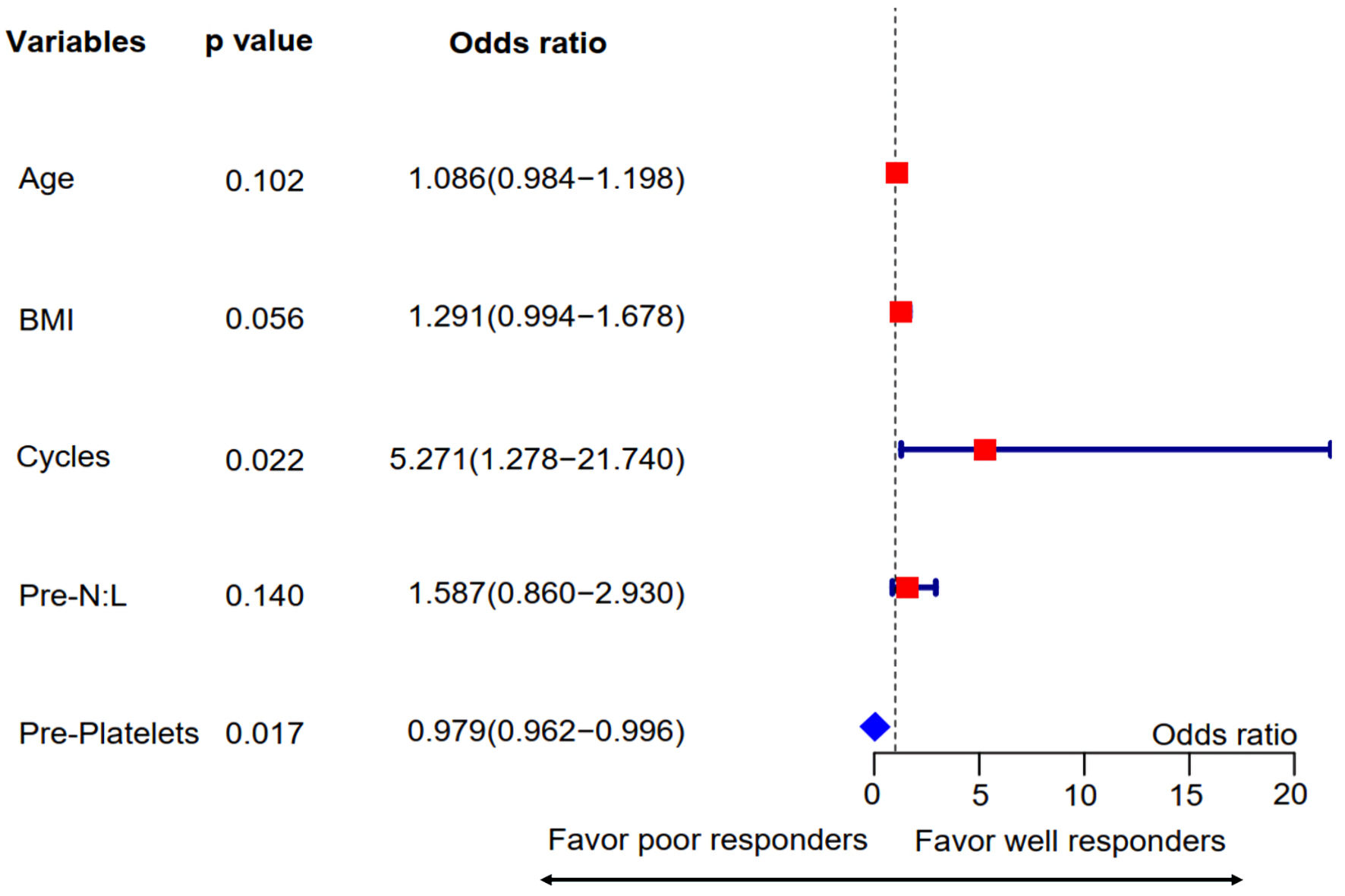 Click for large image | Figure 3. Multivariate regression analysis for pretreatment predictors associated with combined tumor and nodal responses. Variables with P values ≤ 0.1 in the univariate analysis were included in this step. The forest plot showed that treatment cycles and pretreatment platelet counts were independent predictors for combined TN responses. Pre-N:L: pretreatment neutrophil to lymphocyte ratio; Pre-platelets: pretreatment platelet count; BMI: body mass index. |
Prognostic significance of combined pathological responsiveness
We conducted a survival analysis to investigate differences in tumor-associated OS between TN-GRs and TN-PR groups. Significantly higher OS was observed in TN-GRs than that in TN-PRs (P < 0.05, Fig. 4).
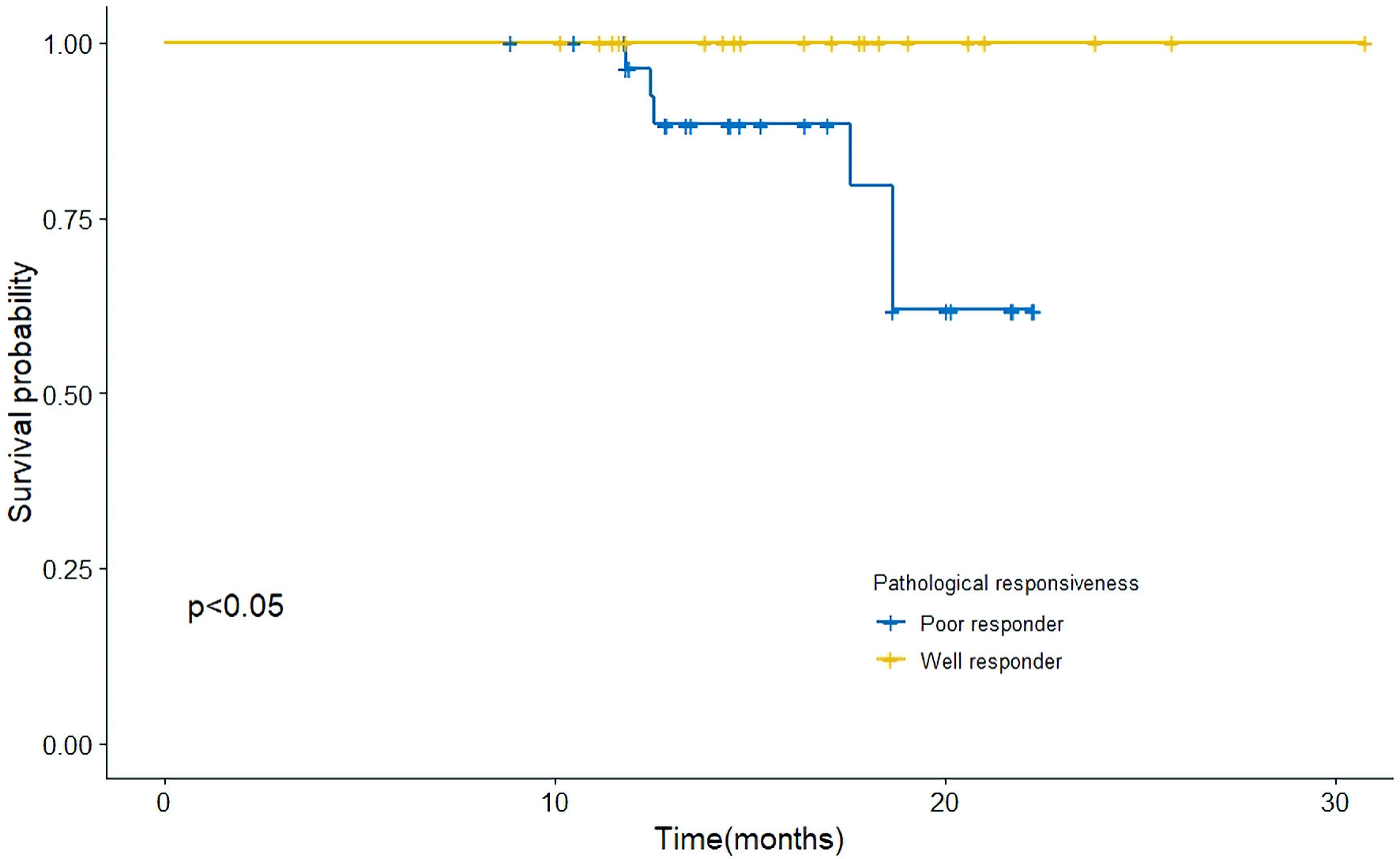 Click for large image | Figure 4. Kaplan-Meier curves for overall survival in the surgical cohort. The difference in OS between pathologically good-responders (TN-GRs) and poor-responders (TN-PRs). Significant difference in overall survival between these groups was observed. OS: overall survival. |
Subgroup survival analysis
Additionally, we conducted survival analyses based on patients’ pretreatment clinical TNM stages. No significant differences in clinical T stage (Supplementary Material 1, www.wjon.org), clinical N stage (Supplementary Material 2, www.wjon.org), and clinical TNM stage (Supplementary Material 3, www.wjon.org) were found.
| Discussion | ▴Top |
Currently, although immunotherapy combined with chemotherapy has not been considered as standard-of-care for ESCC, several studies have proved the satisfying efficacy and safety of NAIC [15-18]. In this pilot study, we described the pathological response of primary tumor and locoregional lymph node metastases of locally advanced ESCC patients receiving NAIC. Based on the differences in response, we classified patients into T-/N-GRs or -PRs. Further, we also identified independent preoperative clinicopathological factors which predicted the overall responses of the primary tumor and regional lymph nodes. To our knowledge, this study was the first to evaluate the patterns of pathological tumor and nodal response after NAIC, and their association with clinicopathological characteristics.
It has been shown that residual viable tumor cells in both primary sites and regional lymph nodes after neoadjuvant therapy were observed to be significantly associated with the prognosis of ESCC [23]. The present study showed significant regressions in the primary tumor after NAIC in most patients with ESCC. The most frequently involved sites were mucosa and submucosa. These findings were consistent with those discovered observed in patients with ESCC receiving neoadjuvant chemoradiotherapy [10, 11]. Notably, 11 patients (21.6%) achieved tumor pathological complete response (T-pCR), which was lower than those reported by recent phase IB-II studies [15-18]. A plausible explanation for this difference is that compared to other clinical trials with idealized designs, this real-world study adopted relaxed inclusion criteria which reflected a more realistic treatment environment.
Subsequently, we classified patients into different groups based on the pathological response of primary tumors and lymph nodes. In terms of T-responders, 76.2% of patients who received three-cycle NAIC achieved a good response, which was twice higher as those who received two-cycle NAIC (P = 0.017). Furthermore, the multivariate binary logistic regression indicated that the neoadjuvant cycle number was an independent predictor for primary tumor response. Those who received three cycles of NAIC were three times more likely to become T-GRs, compared to those that receiving two cycles. A possible explanation for this enhanced efficacy is that an increase in the cycle numbers could lead to an elevated concentration of immunochemotherapy. Moreover, the longer duration of response in immunotherapy-treated groups may be another possible explanation [24, 25]. The three-cycle NAIC could potentially achieve more persistent responses due to a longer neoadjuvant period. Previous studies have reported favorable survival outcomes in tumor patients receiving more cycles of neoadjuvant or adjuvant therapy [26-28]. However, no studies have investigated the differences in the primary tumor response in ESCC patients with varying numbers of neoadjuvant cycles. This study was the first to reveal a relationship between neoadjuvant cycle number and primary tumor response, which could provide a reference for designing trial regimens in the future.
The lymph node response to neoadjuvant therapy was crucial in subsequently followed-up patients with ESCC, especially those with tumor recurrence and metastases [9, 29]. A recent study reported that the predictive values of lymph node response to neoadjuvant therapy were more reliable than those of tumor response [29]. Interestingly, the pattern of lymph node metastases after NAIC was inconsistent with that of the primary tumor. Although lymph node downstaging was observed in 17 (33%) patients, there remain a significant proportion of the patients who had no lymph node response (33%) or even up-staging of the disease (33%). Hamai et al reported that 49.3% of patients had down-staged nodal disease after neoadjuvant chemoradiotherapy. It suggested that the synergistic effect of immunotherapy on chemotherapy may be weaker than on radiotherapy; similar findings were reported by Hsu et al [9]. The distinct response to NAIC between primary tumor and lymph nodes could be attributed to their structural and microenvironmental heterogeneity, such as types and abundance of immune cells, oxygen levels, and cytokines [30-32].
Further, multivariate analyses of the combined primary tumor and lymph nodes responses also indicated that the neoadjuvant cycle number and baseline platelet counts were independent predictors. Patients who received a three-cycle regimen were five times more likely to be TN-GRs compared to those who received a two-cycle regimen. Wu et al reported that no significant association between pretreatment platelet and tumor/nodal responses was observed [33]. Other researchers found that elevation of pretreatment platelets was significantly correlated with better neoadjuvant response, but not OS [34]. However, McLaren et al reported that elevated hematological parameters that incorporate pretreatment platelet count were predictive of worse OS [35]. The discrepancies among these studies may be explained by the high numerical difference in baseline platelets among individuals, which could lead to unstable data. Moreover, the sample sizes in these studies, including the current study (51 - 306), were relatively too small to decisively conclude on the predictive value of the baseline platelet counts. Studies with larger cohorts are needed in the future to confirm this paradoxical observation.
There were some limitations in our study. First, this retrospective study was conducted at a single institution which hampered the clinical promotion and popularization of the findings. Secondly, as discussed earlier, the sample size was too small and large-scale, prospective, multi-centered studies were needed in the future.
In conclusion, we found good response occurred in primary tumors among most ESCC patients receiving NAIC. Good nodal response, on the contrary, only occurred in a small proportion of patients. Furthermore, we identified neoadjuvant cycle number and baseline platelet counts were independent predictors of combined tumor and nodal responses.
| Supplementary Material | ▴Top |
Suppl 1. Kaplan-Meier curves for overall survival in patients with clinical T3 stage and clinical T4a stage.
Suppl 2. Kaplan-Meier curves for overall survival in patients with clinical N0 stage and clinical N+ stage.
Suppl 3. Kaplan-Meier curves for overall survival in patients various clinical TNM stages.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by a grant from the 2020 - 2021 Popularization of Science and Technology Innovation Special Project of Guangdong Province of China (2020A1414070007); the Science and Technology Program of Guangzhou, China (202206010103, 202201011482); and Natural Science Foundation of Guangdong Province (2022A1515012469).
Informed Consent
Informed consent was obtained from all patients.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Shu Jie Huang, Dan Tian and Si Chao Wang: conceptualization, data curation, formal analysis, investigation, methodology, roles/writing - original draft, writing - review and editing. Rui Jie Zeng, Yue Jiao Dong, Liang Li Hong, Han Sheng Wu, Fang Ping Xu, Dong Kun Zhang, Liang Xie, Hai Yu Zhou, Ji Ming Tang, Xiao Song Ben, Gang Chen, Ri Xin Chen: software, validation, visualization, investigation, methodology, roles/writing - original draft; writing - review and editing. Yong Tang and Gui Bin Qiao: funding acquisition, project administration, resources, supervision, roles/writing - original draft, writing - review and editing.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Wang YK, Syu HY, Chen YH, Chung CS, Tseng YS, Ho SY, Huang CW, et al. Endoscopic images by a single-shot multibox detector for the identification of early cancerous lesions in the esophagus: a pilot study. Cancers (Basel). 2021;13(2):321.
doi pubmed - Murphy G, McCormack V, Abedi-Ardekani B, Arnold M, Camargo MC, Dar NA, Dawsey SM, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017;28(9):2086-2093.
doi pubmed - Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132.
doi pubmed - National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers (Version 3.2021). June 22, 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68-74.
doi pubmed - Oguma J, Ozawa S, Koyanagi K, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K. Prognostic significance of pathological tumor response and residual nodal metastasis in patients with esophageal squamous cell carcinoma after neoadjuvant chemotherapy followed by surgery. Esophagus. 2019;16(4):395-401.
doi pubmed - Makino T, Yamasaki M, Tanaka K, Masuike Y, Tatsumi M, Motoori M, Kimura Y, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270(6):1090-1095.
doi pubmed - Hsu PK, Yeh YC, Chien LI, Huang CS, Hsu HS. Clinicopathological significance of pathologic complete lymph node regression after neoadjuvant chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg Oncol. 2021;28(4):2048-2058.
doi pubmed - Shapiro J, ten Kate FJ, van Hagen P, Biermann K, Wijnhoven BP, van Lanschot JJ. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg. 2013;258(5):678-688; discussion 688-679.
doi pubmed - Chao YK, Tsai CY, Chang HK, Tseng CK, Liu YH, Yeh CJ. A pathological study of residual cancer in the esophageal wall following neoadjuvant chemoradiotherapy: focus on esophageal squamous cell carcinoma patients with false negative preoperative endoscopic biopsies. Ann Surg Oncol. 2015;22(11):3647-3652.
doi pubmed - Yamamoto M, Doki Y, Shiozaki H, Yano M, Miyata H, Tamura S, Fujiwara Y, et al. Evaluation of the histologic effect of chemoradiation therapy for squamous cell carcinomas of the esophagus by assessing morphologic features of surgical specimens. Dis Esophagus. 2000;13(4):293-300.
doi pubmed - van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, Meijer SL, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: a single-arm phase II feasibility trial (PERFECT). Clin Cancer Res. 2021;27(12):3351-3359.
doi pubmed - Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, Wu Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. 2021;144:232-241.
doi pubmed - Zhao L, Xing W, Yang Y, Zhang Y, Ma B, Fu X, et al. The sequence of chemotherapy and anti-PD-1 antibody influence the efficacy of neoadjuvant immunochemotherapy in locally advanced esophageal squamous cell cancer: A phase II study. Journal of Clinical Oncology. 2021;39(15_suppl):4051.
doi - Wang Z. Neoadjuvant camrelizumab combined with chemotherapy and apatinib for locally advanced thoracic esophageal squamous cell carcinoma (ESCC): A single-arm, open-label, phase Ib study. Journal of Clinical Oncology. 2021;39(15_suppl):4047.
doi - Li Z, Liu J, Zhang M, Shao J, Yang Y, Li H, et al. A phase II study of neoadjuvant immunotherapy combined with chemotherapy (camrelizumab plus albumin paclitaxel and carboplatin) in resectable thoracic esophageal squamous cell cancer (NICE study): Interim results. Journal of Clinical Oncology. 2021;39(15_suppl):4060.
doi - Li J, Liu J, Li Z, Cui F, Zeng Y, Liang W, et al. Camrelizumab plus chemotherapy as neoadjuvant therapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, open-label, single-arm, phase 2 study. Journal of Clinical Oncology. 2021;39(15_suppl):4028.
doi - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Bottcher K, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98(7):1521-1530.
doi pubmed - Gao Z, Huang S, Tang Y, Wang S, Zhuang W, Ding Y, Wu H, et al. Factors influencing negative surgical outcomes in adult pectus excavatum patients undergoing Nuss procedure. Ann Transl Med. 2021;9(16):1335.
doi pubmed - R Core Team. R: A language and environment for statistical computing. 2020. R Foundation for Statistical Computing: Vienna, Austria.
- Wang X, Wang H, Wang H, Huang J, Wang X, Jiang Z, Tan L, et al. Prognostic value of visual residual tumour cells (VRTC) for patients with esophageal squamous cell carcinomas after neoadjuvant therapy followed by surgery. BMC Cancer. 2021;21(1):111.
doi pubmed - Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830.
doi - Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031.
doi pubmed - Hsieh SF, Lau HY, Wu HH, Hsu HC, Twu NF, Cheng WF. Prognostic factors of early stage epithelial ovarian carcinoma. Int J Environ Res Public Health. 2019;16(4):637.
doi pubmed - Wei J, Feng H, Xiao W, Wang Q, Qiu B, Liu S, Deng M, et al. Cycle number of neoadjuvant chemotherapy might influence survival of patients with T1-4N2-3M0 nasopharyngeal carcinoma. Chin J Cancer Res. 2018;30(1):51-60.
doi pubmed - Zhang RX, Zhou ZG, Lu SX, Lu ZH, Wan DS, Pan ZZ, Wu XJ, et al. Pim-3 as a potential predictor of chemoradiotherapy resistance in locally advanced rectal cancer patients. Sci Rep. 2017;7(1):16043.
doi pubmed - Urakawa S, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y, et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. 2021;273(6):1141-1149.
doi pubmed - Luan S, Zeng X, Zhang C, Qiu J, Yang Y, Mao C, Xiao X, et al. Advances in drug resistance of esophageal cancer: from the perspective of tumor microenvironment. Front Cell Dev Biol. 2021;9:664816.
doi pubmed - Yamamoto K, Makino T, Sato E, Noma T, Urakawa S, Takeoka T, Yamashita K, et al. Tumor-infiltrating M2 macrophage in pretreatment biopsy sample predicts response to chemotherapy and survival in esophageal cancer. Cancer Sci. 2020;111(4):1103-1112.
doi pubmed - Carraro A, Trevellin E, Fassan M, Kotsafti A, Lunardi F, Porzionato A, Dall'Olmo L, et al. Esophageal adenocarcinoma microenvironment: Peritumoral adipose tissue effects associated with chemoresistance. Cancer Sci. 2017;108(12):2393-2404.
doi pubmed - Wu Y, Chen J, Zhao L, Li Q, Zhu J, Yang H, Guo S, et al. Prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma incorporating hematological biomarkers. Cancer Res Treat. 2021;53(1):172-183.
doi pubmed - Ilhan-Mutlu A, Starlinger P, Perkmann T, Schoppmann SF, Preusser M, Birner P. Plasma fibrinogen and blood platelet counts are associated with response to neoadjuvant therapy in esophageal cancer. Biomark Med. 2015;9(4):327-335.
doi pubmed - McLaren PJ, Bronson NW, Hart KD, Vaccaro GM, Gatter KM, Thomas CR, Jr., Hunter JG, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios can predict treatment response to neoadjuvant therapy in esophageal cancer. J Gastrointest Surg. 2017;21(4):607-613.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


