| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 4, August 2022, pages 190-194
Prospective Cohort Study of Palbociclib Treatment in Postmenopausal Patients With Unresectable and Metastatic Hormone Receptor-Positive Breast Cancer: Study Protocol for a CSPOR-BC Palbociclib Cohort Trial
Kazutaka Naruia, f, Takashi Ishikawab, Naruto Tairac, Yukari Uemurad, Hirofumi Mukaie
aDepartment of Breast and Thyroid Surgery, Yokohama City University Medical Center, Yokohama, Japan
bDepartment of Breast Oncology and Surgery, Tokyo Medical University, Tokyo, Japan
cDepartments of General Thoracic Surgery and Breast and Endocrinological Surgery, Okayama University Graduate School of Medicine, Okayama, Japan
dDepartment of Clinical Research Support Center, The University of Tokyo, Tokyo, Japan
eDivision of Oncology/Hematology, National Cancer Center Hospital East, Chiba, Japan
fCorresponding Author: Kazutaka Narui, Department of Breast and Thyroid Surgery, Yokohama City University Medical Center, 4-57 Urafune-cho, Minami-ku, Yokohama 232-0024, Japan
Manuscript submitted June 14, 2022, accepted July 18, 2022, published online August 23, 2022
Short title: Palbociclib Treatment in HR+ MBC
doi: https://doi.org/10.14740/wjon1507
| Abstract | ▴Top |
Background: Combining palbociclib with letrozole or fulvestrant improved progression-free survival in hormone receptor (HR)-positive and human epidermal growth factor receptor-negative metastatic breast cancer. However, combining palbociclib to endocrine treatment increases toxicity and cost compared with the endocrine treatment alone. Moreover, palbociclib treatment may affect the outcome of the subsequent treatment because its benefit in terms of overall survival has not been observed yet. Therefore, it is crucial to examine whether palbociclib can improve clinical outcomes and quality of life (QoL) of patients in the real world.
Methods: A prospective observational study with palbociclib is planned in 3 cohorts (A, B, and C) as per the line of endocrine treatment (i.e., first-line, second-line, or third-line or later-line treatment) for postmenopausal metastatic or unresectable breast cancer. The primary endpoint is progression-free survival in each treatment line, the most commonly used endpoint for global phase 3 studies. As per the results of these studies, the planned sample size is 700 cases: cohort A, 340 cases; cohort B, 200 cases; and cohort C, 130 cases. The secondary endpoints are overall survival, clinical benefit rate, time to chemotherapy, adverse events (AEs), patient-reported outcomes (PROs), and health-related QoL (HRQoL). These endpoints are evaluated again during the subsequent treatment. This study will examine whether the efficacy, safety, and QoL effects of palbociclib treatment in daily clinical practice are not inferior compared to those in clinical trials and whether palbociclib treatment affects the efficacy and safety of the subsequent treatment. Moreover, this study would provide information on the most effective time of adding palbociclib to endocrine treatment.
Discussion: The reproducibility of randomized clinical trials (RCTs) using real-world data must be confirmed to evaluate whether real-world treatment benefits are similar to those observed in RCTs. Although the efficacy of palbociclib has been confirmed in RCTs, Aes of this drug, including its toxicities and cost, are not comparable to those of mono-hormone therapies. Thus, PROs/HRQoL is an important element of this study because several patients with HR-positive metastatic breast cancer have diseases for which sequential hormone therapy is preferential.
Keywords: Breast cancer; Palbociclib; Prospective cohort
| Introduction | ▴Top |
Palbociclib is a first-in-class orally active cyclin-dependent kinase4/6 (CDK4/6) inhibitor that is approved for the treatment of patients with hormone receptor (HR)- positive/human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer. The complex of CDK4/6 and cyclin D1 phosphorylates the retinoblastoma protein (Rb) to relieve repression of E2F-dependent genes and allow progression from the G1 to the S phase cell cycle. They also phosphorylate FOXM1 which activates the expression of other cell-cycle genes, MEP50 which catalyze methylation of p53 signaling pathway with PRMT5, PFK1 and PKM2 which regulate glycolytic enzymes, and SPOP (ubiquitin ligase subunit) that helps with programmed death-ligand 1 (PD-L1) degradation [1, 2]. Palbociclib inhibits the phosphorylation and not only arrests cell cycle directly and indirectly, but also activates immune response.
According to an open-label phase 2 randomized clinical trial (RCT) of PALOMA-1, combining the CDK4/6 inhibitor palbociclib with letrozole significantly improves the progression-free survival (PFS) of patients with metastatic HR-positive/HER2-negative breast cancer, with letrozole as the first-line treatment [3]. This finding was confirmed in the multinational phase 3 RCT of PALOMA-2 [4]. As a second- or later-line treatment for metastatic cancer, combining palbociclib with fulvestrant significantly improves the PFS, as revealed in the phase 3 RCT of PALOMA-3 [5]. Although improvement in the overall survival (OS) has yet to be proven [6], adding palbociclib to existing antihormone agents is consistently beneficial for treating patients with metastatic breast cancer.
However, the incidence of hematologic adverse events (AEs), particularly neutropenia, has been consistently high in palbociclib treatment [3-5]. Although the incidence of febrile neutropenia is lower than expected, the AEs of palbociclib among Asians who are more vulnerable to developing febrile neutropenia than Caucasians should still be investigated [7, 8]. Hematologic AEs are more frequent among the Japanese population than among all cases in both the PALOMA-2 and PALOMA-3 studies [9, 10], but only 46 and 35 cases were enrolled, respectively.
In addition to hematologic AEs, adding palbociclib to endocrine treatment clearly causes increased toxicity and cost compared with endocrine treatment alone. Considering that one important goal for treating metastatic cancer is maintaining patient’s quality of life (QoL), health-related QoL (HRQoL) is included in the PALOMA-2 study [11]. HRQoL is based on the results of patients enrolled in the RCT of the first-line treatment. Furthermore, palbociclib may be used in later-line treatment [12]. Thus, we need to investigate patient-reported outcomes (PROs) or HRQoL in patients who receive each line of treatment in the real world.
The reproducibility of RCTs using real-world data needs to be confirmed, considering the issue as to whether real-world treatment benefits are similar to those observed in RCTs. Real-world data of palbociclib have been used in several studies [12, 13]. However, these studies are all retrospective in design. Thus, we designed a prospective cohort study of palbociclib for postmenopausal metastatic HR-positive breast cancer. This study was planned according to the following two hypotheses: 1) the efficacy, safety, and QoL effects of palbociclib treatment in daily clinical practice are not inferior to those in RCTs during drug development [3-5]; 2) palbociclib treatment affects the efficacy and safety of the subsequent treatment.
| Materials and Methods | ▴Top |
Objective
This study aims to examine whether the efficacy, safety, and QoL effects of palbociclib treatment in daily clinical practice are not inferior to those in RCTs.
Study settings
This study has a prospective multi-institutional cohort study design.
Endpoints
The primary endpoint is the PFS in each line of treatment. PFS is calculated from the date when palbociclib is started to the date when progressive disease is observed or the date of death for any reason. The secondary endpoints are as follows: 1) OS; 2) clinical benefit rate; 3) time from the start of endocrine treatment to chemotherapy; 3) AEs; 4) PROs/HRQoL; 5) PFS, clinical benefit rate, and AEs with the subsequent treatment; 6) PROs with the subsequent treatment; and 7) reasons for selecting the treatment (Fig. 1).
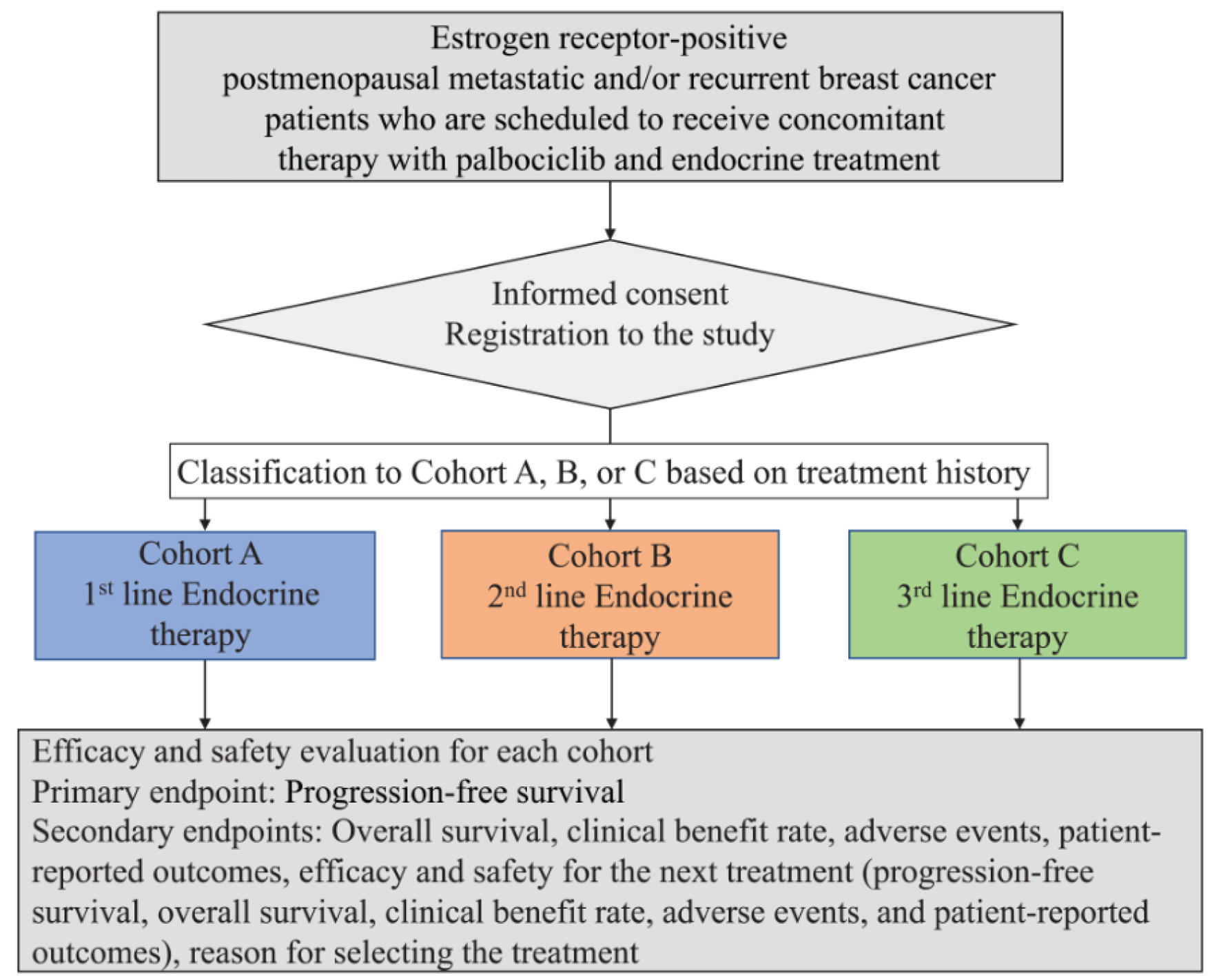 Click for large image | Figure 1. Study design. Patient prospective selection, cohort classification according to the line of endocrine treatment, and treatment efficacy and safety evaluation using primary and secondary endpoints. |
Patients
We have been consecutively and prospectively recruiting patients from designated hospitals in Japan since February 1, 2019, and this recruitment will end on January 31, 2022. We include those postmenopausal women who are diagnosed with unresectable and/or metastatic HR-positive breast cancer and who are planning to undergo first-, second-, and third- or later-line endocrine treatments combined with palbociclib.
We plan three cohorts based on the line of endocrine treatment as follows: 1) cohort A with first-line treatment; 2) cohort B with second-line treatment; and 3) cohort C with third-line or later-line treatment. According to the rationale described subsequently, the planned sample size is 700 cases in which 340, 200, and 130 cases belong to cohorts A, B, and C, respectively.
Treatment methods
Patients undergo palbociclib treatment combined with tamoxifen, fulvestrant, or aromatase inhibitors. Palbociclib is administered once daily for 3 weeks followed by 1 week off in 28-day cycles and only discontinued for reasons such as disease progression, unacceptable toxic effects, study withdrawal, or death. Dose interruptions and reductions are allowed for managing toxic effects.
Assessments
Upon enrollment, we examine the following patient demographic and clinical characteristics: 1) present history (i.e., date of surgery, pathology of surgical specimen including hormone receptor/HER2 status, metastatic site, date of diagnosis on recurrence, and pretreatment at the adjuvant or metastatic setting);2) previous treatment; 3) physical findings (i.e., body mass index and performance status); 4) diagnostic imaging for evaluating breast cancer lesions; 5) laboratory data; 6) AEs before palbociclib treatment, 7) concomitant drugs; and 8) PROs/HRQoL.
Efficacy
Tumors are assessed locally at screening and every 12 weeks by ultrasonography, computed tomography, magnetic resonance imaging, bone scan, or clinical assessment. AEs are assessed in terms of incidence, severity, timing, seriousness, and relatedness to palbociclib. Hematology and blood chemistry tests are conducted and analyzed every 2 weeks for the first two cycles and at the beginning of each cycle thereafter.
AE reporting
AEs are initially confirmed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (Japanese translated, JCOG edition) and are recorded subsequently. Regardless of the grade, we record the presence or absence of the following AEs: constipation, diarrhea, oral mucositis, nausea, vomiting, malaise, pain, joint pain, insomnia, vaginal discharge, vaginal dryness, hot flash, alopecia, and rash. These symptoms are common in breast cancer and are associated with palbociclib-combined endocrine treatment. When other AEs are observed, we grade them as 2 or higher.
PROs and HRQoL
Using patient-reported outcome-common terminology criteria for adverse events (PRO-CTCAE), European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30), and EQ-5D (EuroQol 5 Dimension), we assess the PROs and HRQoL during palbociclib-combined endocrine treatment and the subsequent treatment.
Statistical analysis
The primary endpoint is the PFS because it is commonly and reliably used as the endpoint for phase 3 comparisons in metastatic cancer [2, 3].
Cohort A
In the PALOMA-2 trial, the median PFS for the first-line treatment is 24.8 months [2]. Assuming a median survival time (MST) of 25 months in this study, if 310 cases are collected through a 3-year registration period and a 5-year research duration, the 90% confidence interval of the MST within 8 months or less is 85% probable. Meanwhile, the MST with letrozole monotherapy is 14.5 months. However, considering medical economics, if the threshold to determine the efficacy of this treatment is 20 months of the MST, the lower limit of the confidence interval of the MST will be above the threshold, with 80% statistical power and 310 cases (one-sided, α = 5%).
Cohort B
In the PALOMA-3 trial, the median PFS for the second-line treatment is 9.5 months [3]. Assuming an MST of 9.5 months in this study, if 180 cases are collected through a 3-year registration period and a 5-year research duration, the 90% confidence interval of the MST within 4.5 months or less is 90% probable. Meanwhile, the MST with letrozole monotherapy is 4.6 months. However, considering medical economics, if the threshold to determine the efficacy of this treatment is 7 months of the MST, the lower limit of the confidence interval of the MST will be above the threshold, with 80% statistical power and 180 cases (one-sided, α = 5%).
Cohort C
Although the data to prove the basis for the third-line treatment are limited, the expected MST is 7.5 months. Assuming an MST of 9.5 months in this study, if 120 cases are collected through a 3-year registration period and a 5-year research duration, the 90% confidence interval of the MST within 4 months or less is 85% probable. When 5 months is assumed as the MST of letrozole monotherapy, the lower limit of the confidence interval of the MST will be above the threshold, with 85% statistical power and 120 cases (one-sided, α = 5%). Considering that 10% of the research participants are likely to withdraw, the planned sample sizes are 370, 200, and 130 for cohorts A, B, and C, respectively.
Ethics approval
This study has been approved by the Protocol Review Committee of the Comprehensive Support Project for Oncological Research of Breast Cancer approved this study on March 10, 2018 (approval number T2018-0026) and registered at the UMIN Clinical Trials Registry as UMIN000035863. Ethical approval was obtained from all institutes involved in CSPOR-BC. The study will be conducted in accordance with the legal and regulatory requirements as well as the general principles set forth in the International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organizations of Medical Sciences 2002), Guidelines of Good Clinical Practice (International Conference on Harmonization 1996), and the Declaration of Helsinki (World Medical Association 1996 and 2008).
| Discussion | ▴Top |
The AEs of palbociclib, including its toxicities and cost, are not comparable to those of hormone monotherapies, although several RCTs confirmed its efficacy [3-6]. Thus, PROs/HRQoL is important in this study, considering that numerous patients with HR-positive metastatic breast cancer have diseases for which sequential hormone therapy is preferential. The optimal treatment strategy for palbociclib as a first-line therapy still remains unknown. An important RCT that addresses this issue is the SONIA study [14]. We plan to investigate the duration of the prior and subsequent treatments in the first-line and second-line cohorts to compare their time from the start of the endocrine treatment to chemotherapy for metastatic diseases. Therefore, our study potentially addresses the same issue as that of the SONIA study.
In conclusion, this prospective study aims to investigate the efficacy and safety of palbociclib in the real world and to clarify whether palbociclib is the drug of choice as first-line treatment in all patients with metastatic HR-positive diseases in daily clinical practice.
Trial status
This study started recruiting patients from February 1, 2019 and will continue recruitment until January 31, 2022 from designated hospitals in Japan. The protocol version is 1.0, which was established on October 3, 2018.
Acknowledgments
We thank Dr. Edward Barroga (https://orcid.org/0000-0002-8920-2607), Medical Editor and Professor of Academic Writing at St. Luke’s International University for editing the manuscript.
Financial Disclosure
The funding for this research is based on the stipulated contract between Pfizer Inc. (grant number: WI235404) and Comprehensive Support Project for Oncological Research of Breast Cancer (grant number: grant from CSPOR-BC: BC09). Pfizer Inc. is not involved in the decision-making process for any activities.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All eligible patients granted written informed consent.
Author Contributions
TI and KN conceived, designed, interpreted data, and drafted the manuscript. NT assisted QoL study and paper revisions. YU completed analyses for trial data. HM supervised the overall study and approved the submission of the study design to the journal as the representative of CSPOR-BC. All authors read and approved the manuscript.
Data Availability
Data supporting the trial will be made available on the CSPOR-BC website (https://cspor-bc.or.jp). Detailed datasets for this trial will be kept in the data center of the CSPOR-BC office and will not be accessible during data collection. At trial conclusion, summary data will be made available on the website to aid interpretation and replication of analyses.
Author Note
The authors belong to CSPOR-BC, a clinical trial group studying breast cancer in Japan since 2000. The authors have managed and disseminated the data of various clinical trials from Japan.
Abbreviations
AE: adverse event; CTCAE: Common Terminology Criteria for Adverse Events; HR: hormone receptor; HRQoL: health-related quality of life; OS: overall survival; PRO: patient-reported outcome; PFS: progression-free survival; QoL: quality of life; RCT: randomized clinical trial
| References | ▴Top |
- Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18(1):17.
doi pubmed - Alvarez-Fernandez M, Malumbres M. Mechanisms of sensitivity and resistance to CDK4/6 inhibition. Cancer Cell. 2020;37(4):514-529.
doi pubmed - Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35.
doi - Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936.
doi pubmed - Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439.
doi - Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, et al. Overall Survival with Palbociclib and Fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926-1936.
doi pubmed - Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, Teramukai S, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27(21):3540-3546.
doi pubmed - Han HS, Reis IM, Zhao W, Kuroi K, Toi M, Suzuki E, Syme R, et al. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Cancer. 2011;47(17):2537-2545.
doi pubmed - Mukai H, Shimizu C, Masuda N, Ohtani S, Ohno S, Takahashi M, Yamamoto Y, et al. Palbociclib in combination with letrozole in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-2 subgroup analysis of Japanese patients. Int J Clin Oncol. 2019;24(3):274-287.
doi pubmed - Masuda N, Inoue K, Nakamura R, Rai Y, Mukai H, Ohno S, Hara F, et al. Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int J Clin Oncol. 2019;24(3):262-273.
doi pubmed - Rugo HS, Dieras V, Gelmon KA, Finn RS, Slamon DJ, Martin M, Neven P, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol. 2018;29(4):888-894.
doi pubmed - Kish JK, Ward MA, Garofalo D, Ahmed HV, McRoy L, Laney J, Zanotti G, et al. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res. 2018;20(1):37.
doi pubmed - Varella L, Eziokwu AS, Jia X, Kruse M, Moore HCF, Budd GT, Abraham J, et al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res Treat. 2019;176(2):429-434.
doi pubmed - van Ommen-Nijhof A, Konings IR, van Zeijl CJJ, Uyl-de Groot CA, van der Noort V, Jager A, Sonke GS, et al. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer - the SONIA study: study protocol for a randomized controlled trial. BMC Cancer. 2018;18(1):1146.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


