| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 4, August 2022, pages 222-234
Sialidase NEU1 May Serve as a Potential Biomarker of Proliferation, Migration and Prognosis in Melanoma
Qiu Penga, g, Liang Gaob, g, Hong Bing Chengc, d, g, Jin Sheng Wangc, e, h, Jia Wangc, f, h
aHunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410013, China
bDepartment of Dermatology, Heji Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi 046000, China
cCollaborative Innovation Center for Aging Mechanism Research and Transformation, Center for Healthy Aging, Changzhi Medical College, Shanxi 046000, China
dDepartment of Microbiology, Changzhi Medical College, Changzhi, Shanxi 046000, China
eKey Laboratory of Esophageal Cancer Basic Research and Clinical Transformation, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, Shanxi 046000, China
fDepartment of Immunology, Changzhi Medical College, Changzhi, Shanxi 046000, China
gThese authors contributed equally to this study.
hCorresponding Author: Jia Wang, Department of Immunology, Changzhi Medical College, Changzhi, Shanxi 046000 China; Jin Sheng Wang, Collaborative Innovation Center for Aging Mechanism Research and Transformation, Center for Healthy Aging, Changzhi Medical College, Shanxi 046000, China
Manuscript submitted June 22, 2022, accepted August 6, 2022, published online August 23, 2022
Short title: NEU1 Promotes Melanoma Progression
doi: https://doi.org/10.14740/wjon1509
| Abstract | ▴Top |
Background: Melanoma is a kind of malignant tumor with high mortality originating from melanocytes. It is urgent to find new molecular biomarkers for prognosis and new treatment methods for melanoma. As an important molecule of sialidase family, neuraminidase-1 (NEU1) has been found to play an important role in regulating the occurrence and progression of tumors, but the role of NEU1 in melanoma is not sure.
Methods: The expression level of NEU1 in melanoma and normal tissues was evaluated by analyzing the expression data from ONCOMINE, UALCAN and GEPIA database. The mutation, copy number alteration and gene correlation of NEU1 in melanoma were evaluated by analyzing the melanoma data from cBioPortal database The protein expression levels of NEU1 were further validated by immunohistochemical (IHC) staining data from The Human Protein Atlas database. The melanoma data in TIMER 2.0 database were used to analyze the correlation between NEU1 expression and immune cell infiltration. The proliferative and migratory abilities of melanoma cells were examined by cell proliferation and migration assay in vitro and nude mice.
Results: We discovered that NEU1 was highly expressed in melanoma samples compared with normal samples. The alteration frequency of NEU1 in melanoma patients reached 18%, and most of them were “mutation” type. The expression of NEU1 was positively correlated with the overall survival of patients with melanoma. The expression of NEU1 was positively correlated with the expression of proliferation marker CDK2 and epithelial-mesenchymal transition marker CD44 and negatively correlated with the expression of apoptosis marker CASP3 and CASP8. Moreover, the expression level of NEU1 was related to the infiltration of immune cells in melanoma. Knockdown of NEU1 attenuated the in vitro proliferative and migratory abilities of melanoma cells, as well as in vivo tumor progression of melanoma cells.
Conclusions: These findings suggest that NEU1 may play a key role in the development of melanoma and may be used as a prognostic target of melanoma.
Keywords: NEU1; Melanoma; Invasion; Tumorigenesis; Survival
| Introduction | ▴Top |
Melanoma is a kind of skin tumor that is caused by the malignant transformation of melanocytes, and its morbidity and mortality rate are both on the rise. Although surgical resection can effectively treat local melanoma, the prognosis of metastatic melanoma is still poor [1, 2]. The uncontrollable changes of multiple signal pathways related to cell cycle and the dysregulation of apoptosis are the result of malignant proliferation of melanocytes. The most important way is to activate RAS/ERK/MAPK signaling through BRAF or NRAS site mutations, which leads to cell canceration [3, 4]. In about 50% of melanomas, the BRAF gene has activated mutations, and 90% of them encode the active BRAF V600E protein [5, 6]. In recent years, the development of BRAF inhibitors and immune checkpoint inhibitors can effectively inhibit the tumor progression for some malignant melanoma patients, thereby improving the survival of patients [7, 8]. However, a considerable number of patients are insensitive to treatment or have drug resistance [9, 10]. At present, although there are many treatment strategies for melanoma, such as surgical resection, chemotherapy, radiotherapy and immunotherapy, the current annual incidence of melanoma patients in the world is still high. For patients with metastatic melanoma, the overall 5-year survival rate is only about 15% [11], so it is urgent to find new prognostic molecular targets and new melanoma treatment methods. Cancer-targeted therapy research has made breakthrough progress in recent years, but effective therapeutic intervention targets for melanoma and its clinical application are still very limited, and it has not fundamentally changed the current high mortality rate of melanoma. Therefore, finding new and effective targets for the occurrence and development of melanoma has important practical significance for the diagnosis and treatment of melanoma, and improving the survival rate and quality of life of patients.
Neuraminidase-1 (NEU1), also known as the lysosomal neuraminidase gene, encodes a protein called lysosomal enzyme, which forms a lysosomal multienzyme complex with PPCA and β-GAL, and is expressed in many tissues and cells. In mammalian cells, NEU1 catalyzes sialic acid residues, further triggers the degradation of sugars, and participates in cellular message transmission in immune response [12]. Currently, four kinds of sialidases NEU1, NEU2, NEU3 and NEU4 have been found in mammals. The former three are distributed in lysosome, cytoplasm, and plasma membrane, respectively, while NEU4 is located in lysosome, mitochondria and cell inner membrane [13]. From an evolutionary perspective, NEU1 has the highest conservation and expression level in all mammalian tissues. Its main function is to specifically recognize and cut the sialic acid residues at the modified end of the sugar chain on oligosaccharides and glycoproteins. Compared with the other three sialidases, NEU1 is associated with inflammatory response, Alzheimer’s disease, cancer, and diabetes [14-17]. NEU1 has been found to play a key role in tumors in recent years. For example, after NEU1 is overexpressed in colon cancer cell HT29, its ability to metastasized to the liver is significantly affected when injected into the spleen of mice [18]. NEU1 can remove the sialylation modification on integrin β4, resulting in a decrease in its phosphorylation, which in turn inhibits the activity of FAK kinase and the ERK1/2 signaling, thereby affecting tumors migration [19]. In addition, some studies have reported that the NEU1 inhibitor can inhibit the progression of drug-resistant pancreatic cancer cells [20]. These findings indicate that NEU1 plays a regulatory role in metastasis, invasion, and adhesion of cancer, and may provide a new means for the treatment of cancer.
Here, we used The Cancer Genome Atlas (TCGA) and several public databases to explore the expression and mutation of NEU1 in melanoma. We found that NEU1 was highly expressed in melanoma, and the higher expression of NEU1, the worse the overall survival rate of melanoma patients. We analyzed the NEU1-related genomic changes and functional networks in melanoma and evaluated their role in tumor immunity through multidimensional analysis. We also demonstrated that NEU1 can promote the proliferation and metastasis of melanoma cells in vivo and in vitro. These results suggest that NEU1 may play a key role as a biomarker for melanoma in tumorigenesis and development.
| Materials and Methods | ▴Top |
Cell culture and transfection of siRNA
A857 human melanoma cell line was cultured in minimum essential medium (MEM) medium containing 10% fetal bovine serum. The siRNA of NEU1 (si-NEU1) was synthesized from Ribobio company (Guangzhou, China). si-NEU1 was transfected into A857 cells with Lipofectamine 3000 (Invitrogen) transfection reagent.
Bioinformatics analysis
ONCOMINE database
The differential expression of NEU1 in melanoma and normal tissues was analyzed by using the melanoma-related microarray data in ONCOMINE database.
UALCAN database
We used the UALCAN database to analyze the expression level of NEU1 in normal tissues, primary melanoma and metastatic melanoma respectively, by entering “NEU1” [21].
GEPIA database
The GEPIA database was used for interactive analysis of gene expression profile of tumors and normal tissues [22]. Here, we analyzed the differential expression of NEU1 in melanoma and normal samples by using GEPIA database.
TIMER database
The melanoma data in TIMER 2.0 database were used to analyze the correlation between NEU1 expression and immune cell infiltration [23]. In this study, we analyzed the correlation between NEU1 expression and the infiltration of seven types of immune cells: CD8+ T cells, CD4+ T cells, B cells, regulatory T (Treg) cells, macrophages, dendritic cells, and natural killer (NK) cells.
cBioPortal database
The mutation, copy number alteration and gene correlation of NEU1 in melanoma were analyzed by using the melanoma data in cBioPortal database [24, 25].
The Human Protein Atlas database
By using pathological samples from The Human Protein Atlas database, we analyzed the protein expression level of NEU1 in melanoma samples and corresponding normal tissues.
Cell proliferation assay of human melanoma cells
The effect of NEU1 differential expression on the proliferation of melanoma cells was detected by plate clone formation assay. Differentially expressing NEU1 A857 human melanoma cells line was inoculated into six-well plates and cultured in medium for 10 days. Then the cells were washed with phosphate buffered saline (PBS), fixed with paraformaldehyde and stained with viola crystalline. Finally, the number of clones was estimated by Image J software after microscope photography.
Cell migration assays
The migration ability of melanoma cells with different expression of NEU1 was analyzed by Transwell Matrigel experiment. Differentially expressing NEU1 A857 human melanoma cells line (1 × 105) resuspended at 200 µL was planted in the upper chamber in serum-free medium; then it was added with 500 µL in the lower room medium supplemented with 10% FBS. After 12 h, the migration number of melanoma cells differentially expressing NEU1 was detected.
Animal experiment
All animal care and euthanasia protocols were approved by the Institutional Animal Care and Use Committee of Changzhi Medical School (Changzhi, China). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). For the melanoma xenograft study, 3 - 4 weeks old nude mice were implanted into the right flank of mice subcutaneously with 5 × 106 A857 cells transfected with control (NC) or stably knockdown NEU1 (sh-NEU1) in 100 µL MEM. After 1 month, the mice were dissected, and the tumor tissue was removed for fixation, dehydration, and photographs.
Statistical analysis
GraphPad Prism 8 software was used for statistical analysis. The data were expressed as mean ± standard deviation, and the differences between groups were assessed by unpaired Student’s t-test. P values < 0.05 were considered significant.
| Results | ▴Top |
NEU1 was highly expressed in melanoma
To explore the function of NEU1 in melanoma, we constructed a screening strategy: significant analysis of melanoma tissues GEO data was carried out to analyze the differentially expressed genes (including melanoma biopsies and normal tissues from three sets of microarray data (#GSE96887, GSE35388 and GSE3189) (Fig. 1a-c). We first detected the upregulated gene expression in three sets of melanoma microarray data of and found that NEU1 was not only the most significantly increased gene in melanoma tissues, but also the only most significantly upregulated gene in three GEO datasets (Fig. 1d).
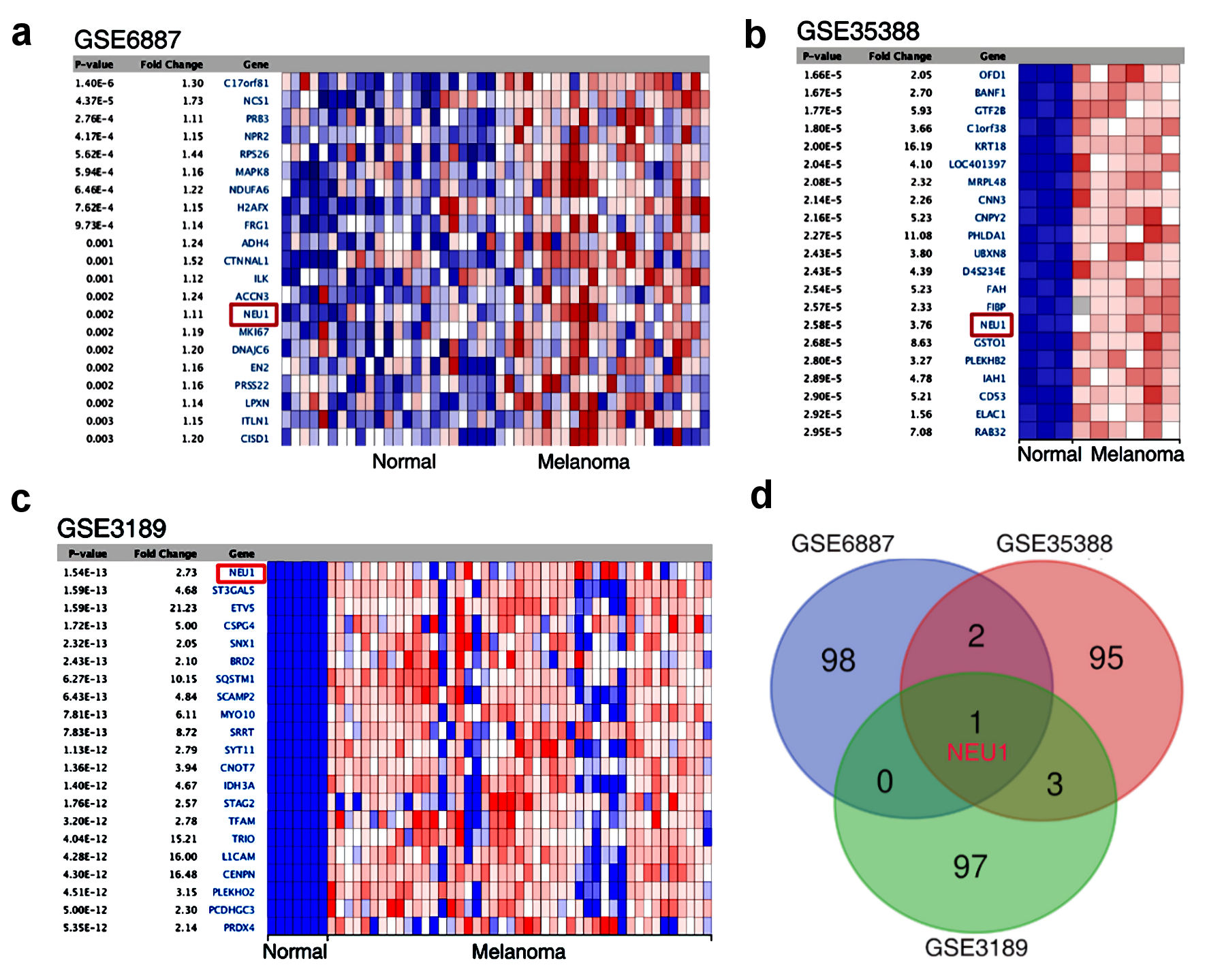 Click for large image | Figure 1. NEU1 was highly expressed in melanoma. (a-c) Heatmap showing genes that are dysregulated in melanoma compared to normal tissues mined from GSE6887, GSE35388 and GSE3189. (d) Venn diagram identified and validated the dysregulated expression genes shared in the three GEO data sets. NEU1: neuraminidase-1. |
NEU1 expression correlates with the prognosis of patients with melanoma
To further explore the relationship between NEU1 and the occurrence and development of melanoma, we used public data from ONCOMINE database to analyze the expression of NEU1 in melanoma samples [26]. We found that the expression of NEU1 in melanoma was significantly higher than that in normal tissues (Fig. 2a) [27-29]. Through the TCGA database [30], we further confirmed that the expression of NEU1 in melanoma samples was significantly higher than that in normal samples (Fig. 2b). Moreover, the expression of NEU1 in the metastatic melanoma tissues was significantly higher than that in primary melanoma samples, indicating that NEU1 may be related to the metastasis of melanoma (Fig. 2c). Consistent with the previous public database results, we used the immunohistochemical results of melanoma pathological samples in The Human Protein Atlas database to confirm that the protein level of NEU1 in melanoma patients was higher than that in normal tissues (Fig. 2d). The correlation between NEU1 expression and overall survival was analyzed by using the gene expression data of melanoma patients. Patients with higher NEU1 expression had shorter overall survival than those with lower NEU1 expression (Fig. 2e).
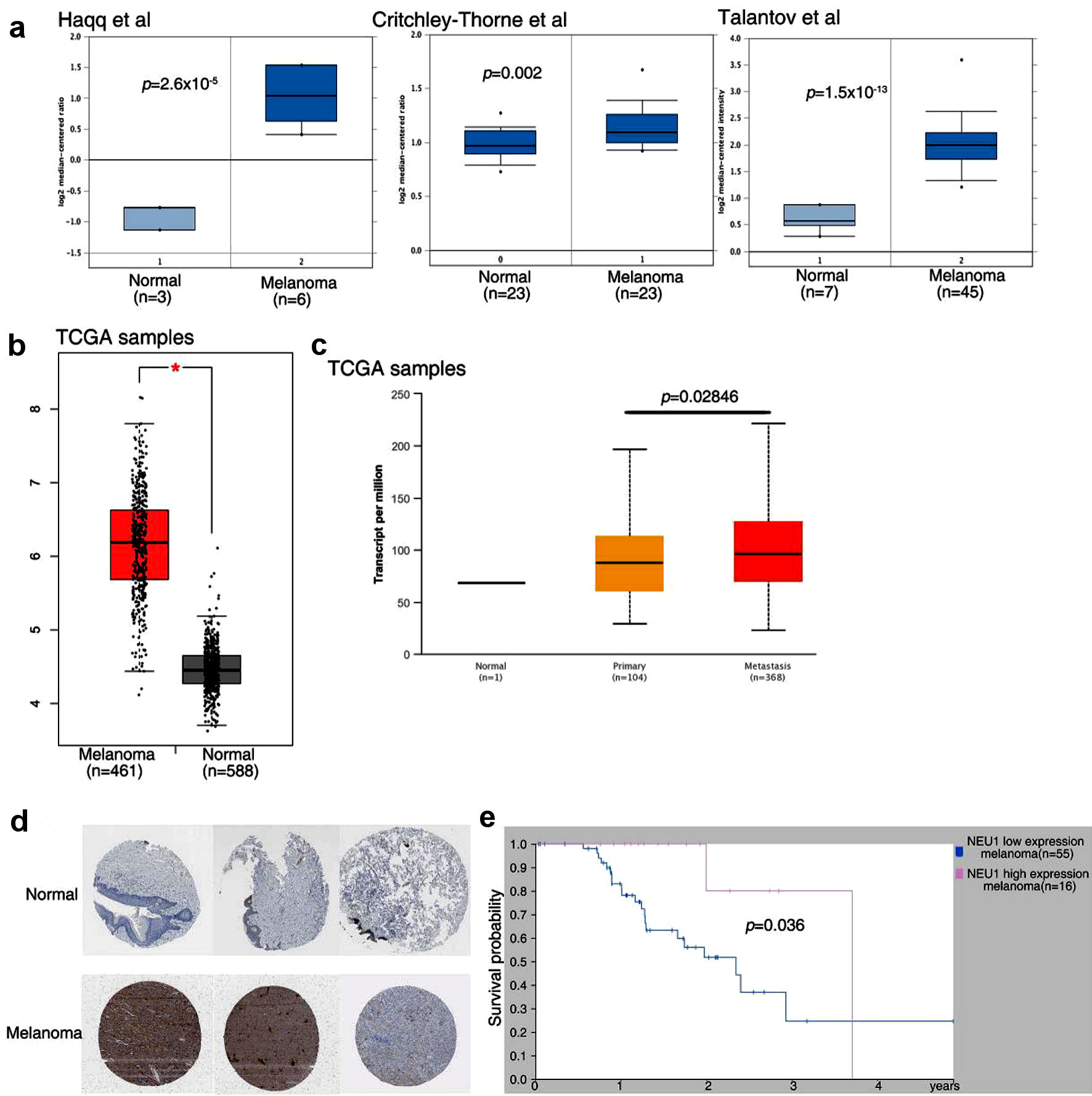 Click for large image | Figure 2. NEU1 expression in melanoma is associated with poor patient survival. (a) Expression data of NEU1 from ONCOMINE database in melanoma samples and controls. (b) Expression data of NEU1 from GEPIA database in melanoma samples. (c) NEU1 expression data in non-cancer controls, primary cancer samples and metastasis melanoma samples from UALCAN database. (d) NEU1 expression data from The Human Protein Atlas database in melanoma patient with or non-cancer controls were measured by immunohistochemical staining. (e) Overall survival rate of melanoma patients in high expression NEU1 group and low expression NEU1 group in The Human Protein Atlas database. NEU1: neuraminidase-1; TCGA: The Cancer Genome Atlas. |
Genetic alteration of NEU1 in melanoma
We analyzed the genetic changes of NEU1 in melanoma using cBioPortal database. As shown in Figure 3a, the alteration frequency of NEU1 in melanoma patients reached 18%, and most of them were “mutation” type. Interestingly, most of the melanoma patients with NEU1 genetic changes have high expression of NEU1 mRNA (Fig. 3a). Figure 3b further shows the types and sites of NEU1 gene mutations. We found that the main type of NEU1 genetic change is missense mutation. The R341 mutation of BNR2 domain was detected in three cases of melanoma (Fig. 3b), which could induce the frame shift mutation of NEU1 gene. Moreover, we further found that fraction genome altered of NEU1 is significantly positively correlated with mRNA expression of NEU1 (Fig. 3c). In addition, we also investigated the correlation between NEU1 mutations and clinical survival and prognosis in patients with melanoma. Figure 3d shows that the overall survival and prognosis of melanoma patients without NEU1 mutation are better (P = 0.00129), compared with cases with NEU1 alteration. We also explore the correlation between NEU1 expression level and the tumor-associated markers, such as proliferation markers cyclin-dependent kinase 2 (CDK2), epithelial-mesenchymal transition markers CD44 and apoptosis marker CASP3 and CASP8. We analyzed the cBioPortal database (http://www.cbioportal.org/) and showed that in melanoma samples, the expression of NEU1 was positively correlated with the expression of CDK2 and CD44 and negatively correlated with the expression of CASP3 and CASP8 (Fig. 3e). These results suggest that NEU1 may be involved in the regulation of melanoma proliferation and metastasis by regulating the expression of these related markers.
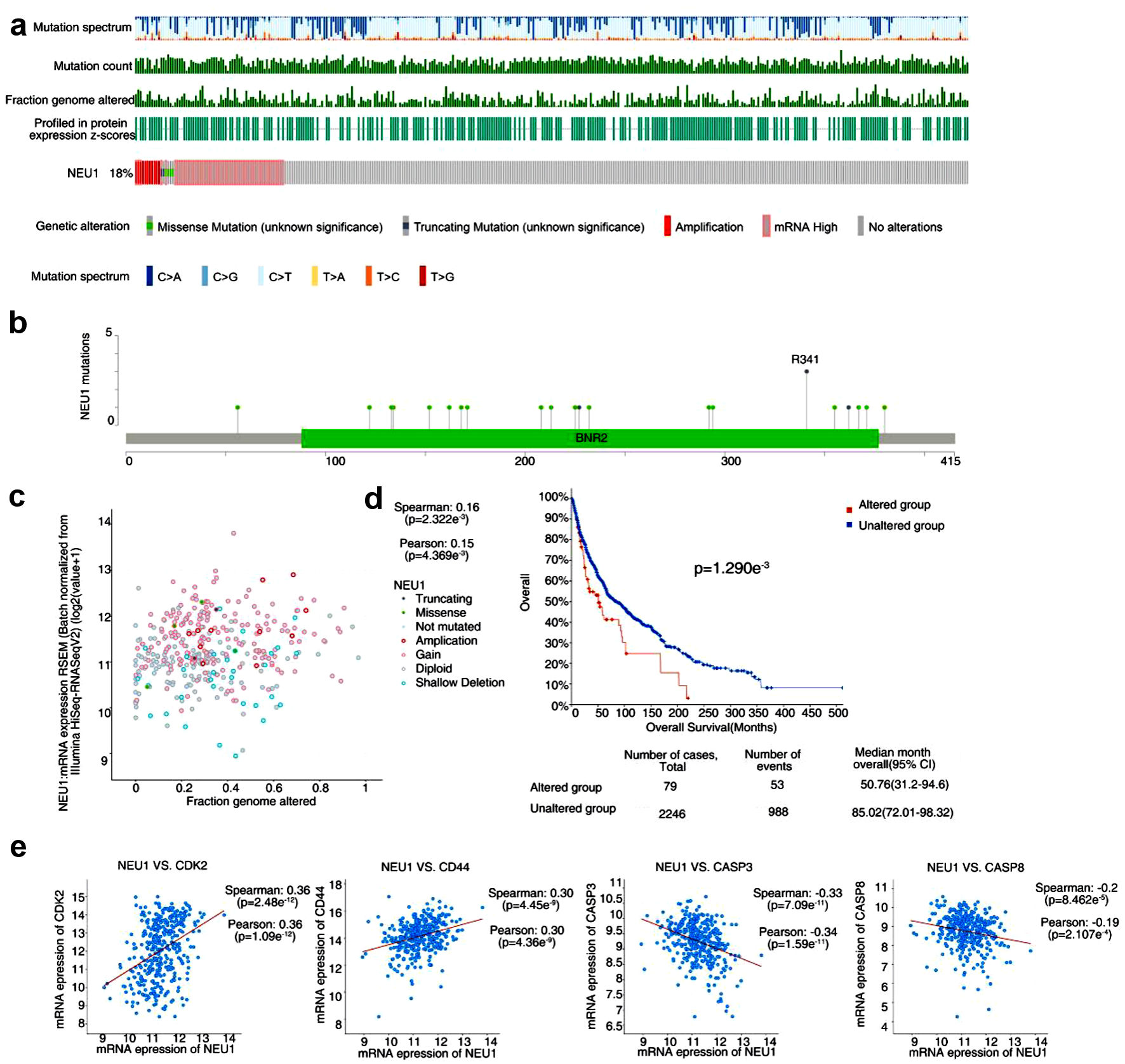 Click for large image | Figure 3. Genetic alteration of NEU1 in melanoma. (a) NEU1 genetic alterations in the melanoma using the cBioPortal tool. (b) The mutation site and mutation frequency of NEU1 were displayed using the cBioPortal tool. (c) Correlation analysis of fraction genome altered of NEU1 with mRNA expression of NEU1 using the cBioPortal tool. (d) Correlation between NEU1 mutation and overall survival in melanoma. (e) Correlation between expression of NEU1 in melanoma and tumor related markers such as CDK2, CD44, CASP3 and CASP8. NEU1: neuraminidase-1. |
Relationships of NEU1 and immune cells
Tumor infiltrating immune cells are a key part of tumor microenvironment, which are closely related to the progression of tumor [31, 32]. We analyzed the correlation between the expression level of NEU1 and immune cell infiltration by using TIMER 2.0 database. Figure 4a shows the correlation between the expression of NEU1 and the number of seven tumor infiltrating immune cell types, including CD4+ T, CD8+ T, B cells, Treg cells, macrophages, NK cells, and dendritic cells. We found that the expression levels of NEU1 in melanoma were significantly correlated with the abundance of tumor-infiltrating immune cells after adjustment for purity (Fig. 4a). In order to investigate the correlation between the degree of immune cell infiltration and the prognosis of patients with melanoma, we used Kaplan-Meier Plotter method to analyze the relationship between the abundance of immune cell infiltration and prognosis. We found that in patients with melanoma, the abundance of infiltration of CD4+ T, CD8+ T, B, Treg, macrophages, NK, and dendritic cells in the melanoma is significantly correlated with the patient’s survival (Fig. 4b).
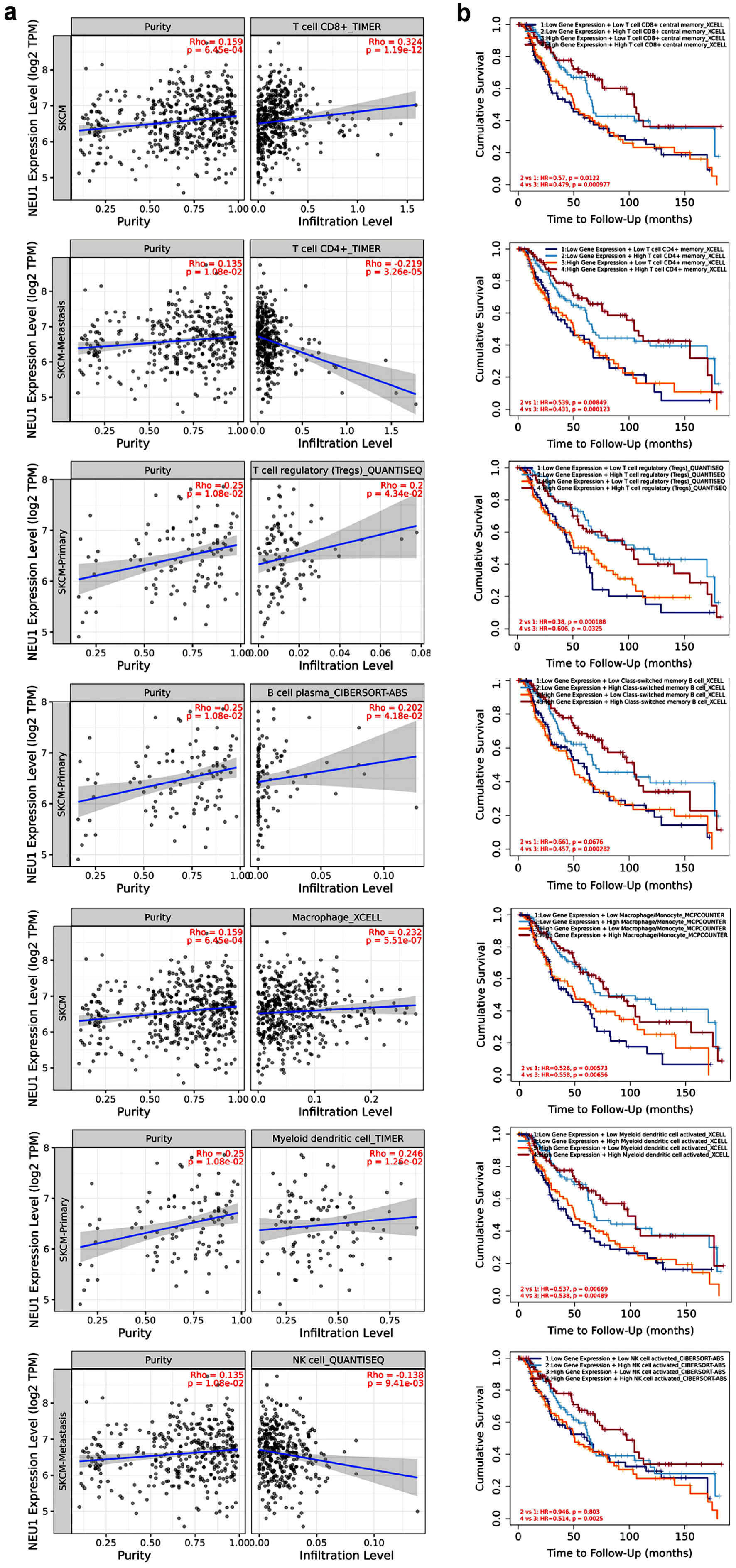 Click for large image | Figure 4. Correlation between NEU1 and immune cell infiltration. (a) The correlation between NEU1 expression and the infiltration of CD4+ T cell, CD8+ T cell, B cell, regulatory T (Treg) cell, macrophage, and natural killer (NK) cell in melanoma was analyzed by TIMER 2.0 tool. (b) The relationship between the immune cell infiltration and the overall survival of melanoma using the TIMER 2.0 tool. NEU1: neuraminidase-1. |
Enrichment analysis of NEU1-associated partners
In order to further explore the potential molecular mechanism of NEU1 regulating melanoma progression, we performed a series of pathway enrichment analysis on NEU1 expression-related genes. By using the UALCAN database (http://ualcan.path.uab.edu/index.html), we screened the genes significantly related to NEU1 expression in normal tissues, primary melanoma, and metastatic melanoma. We mapped the top 20 genes with positive and negative correlation according to the value of correlation coefficient with NEU1 expression (Fig. 5a, b). Moreover, we also explored the possible function and regulatory signal pathway of NEU1 in melanoma by GO and KEGG analysis of NEU1 expression-correlated genes. Figure 5c and d respectively shows the GO enrichment results and KEGG enrichment results of NEU1 expression-related genes, which indicated that “cell cycle”, “DNA replication”, “autophagy” and “ERK signaling pathway” were the higher enrichment functions related to NEU1 in melanoma (Fig. 5c). Figure 5d shows that “protein processing”, “spliceosome”, “metabolic pathway” and “lysosome” are the first four signaling pathways associated with NEU1 in melanoma.
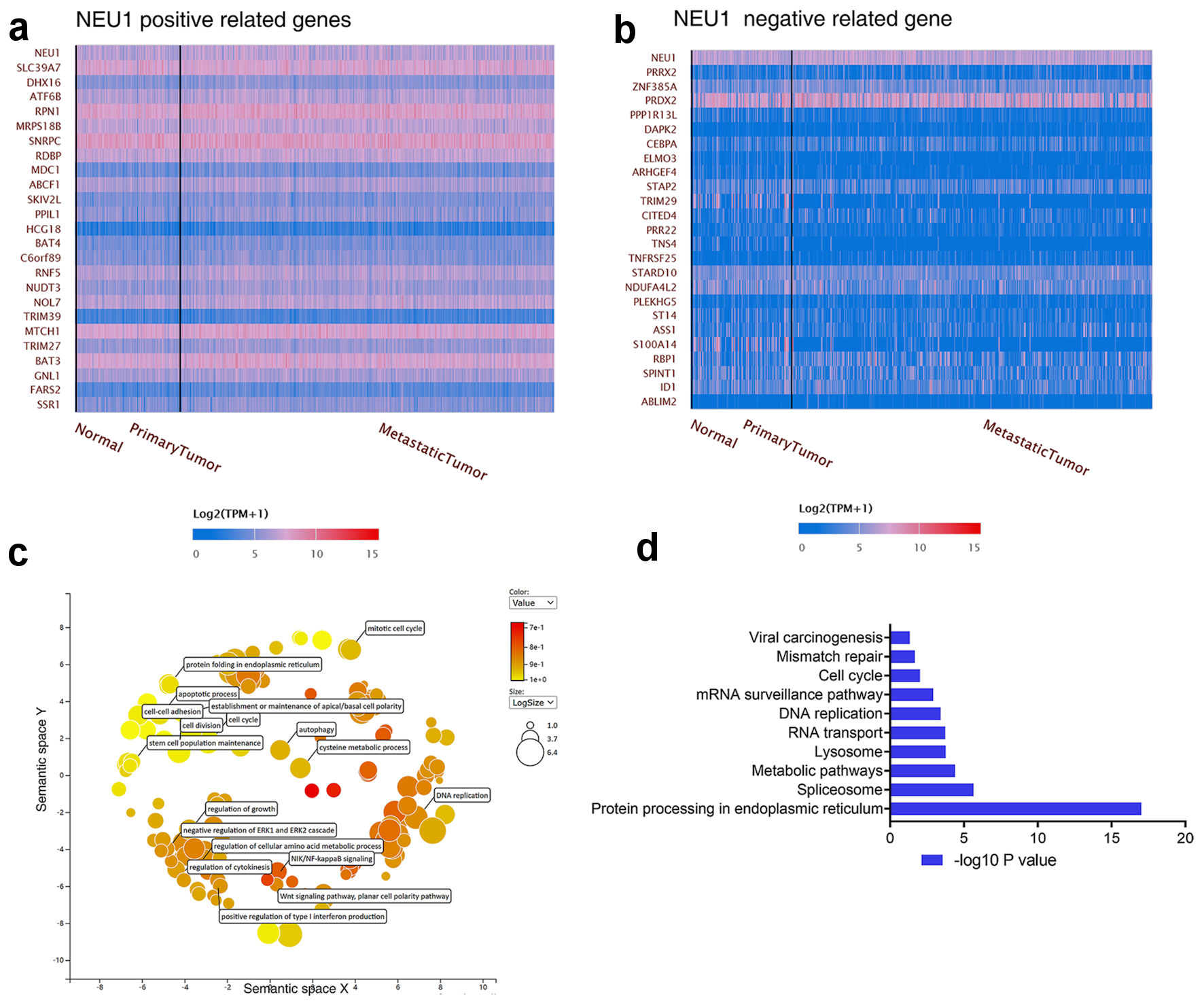 Click for large image | Figure 5. Enrichment analysis of NEU1-related partners. (a, b) The top 20 genes with positive correlation (a) and negative correlation (b) with NEU1 in melanoma were analyzed in the UALCAN database. (c) Functional enrichment histogram of important modules by using REVIGO tools. (d) Pathway enrichment map of NEU1 and NEU1-correlated genes. NEU1: neuraminidase-1. |
NEU1 promotes melanoma cell growth and migration
To assess the functional role of NEU1 in melanoma cells, we transfected overexpression vector or si-NEU1 into A875 cells. The tumor cell clone formation assay revealed that overexpression of NEU1 significantly promoted the cell growth of melanoma cells, whereas knockdown of NEU1 inhibited cell growth (Fig. 6a). To detect roles of NEU1 in cell migration, we conducted transwell assays in melanoma cells. As expected, NEU1 overexpression significantly promoted the migration in the A875 cells (Fig. 6b). Knocking down NEU1 in A875 cell line can significantly inhibit the migration abilities of the cells. Collectively, these data suggested that NEU1 promotes melanoma growth and migration in vitro.
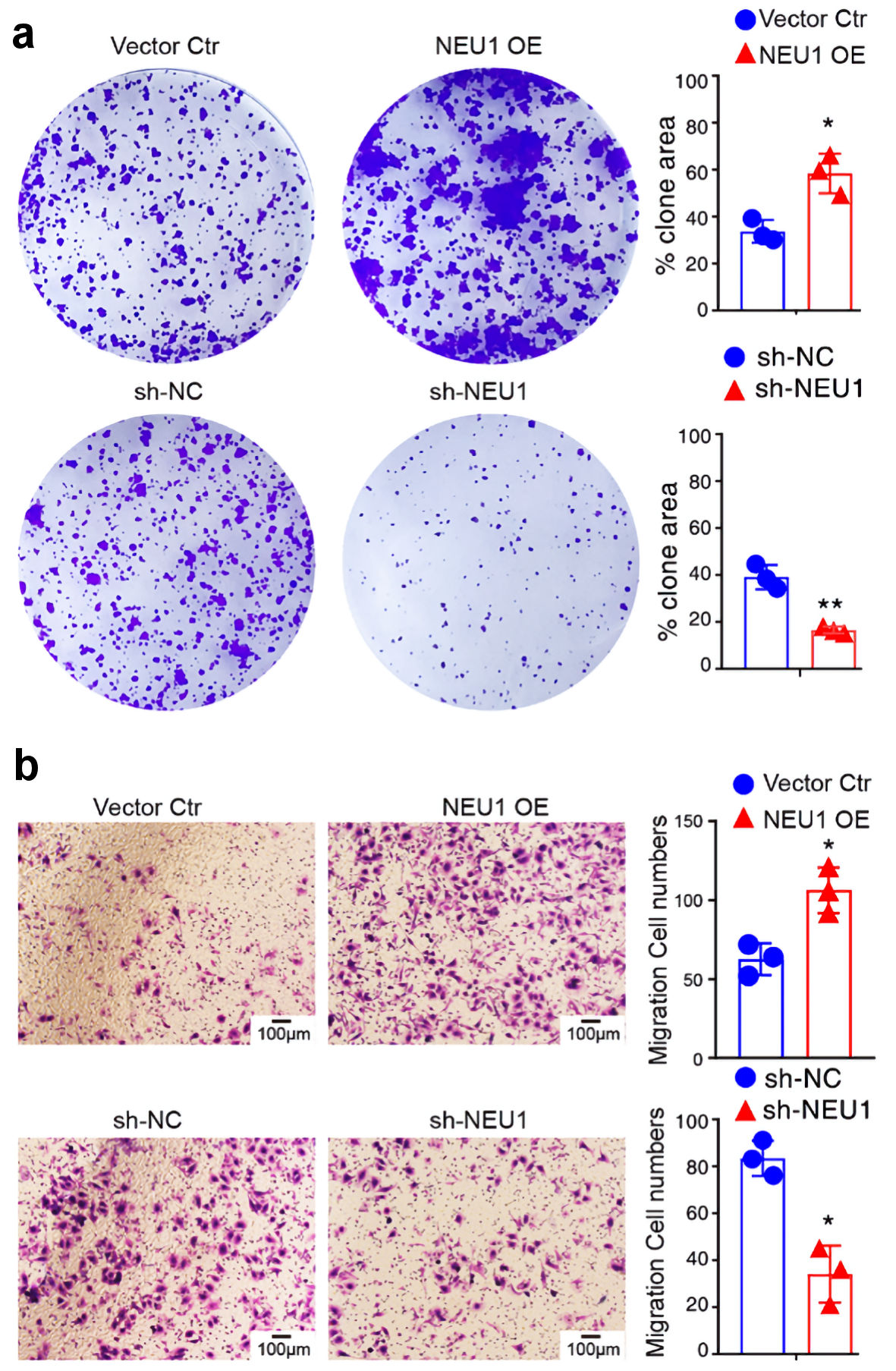 Click for large image | Figure 6. NEU1 promotes melanoma cells growth and migration. (a) Colony formation of A875 cells 2 weeks after transfection with the indicated vector. (b) Transwell assay was used to detect the effect of NEU1 on the migration of A875 cells. NEU1: neuraminidase-1. NEU1: neuraminidase-1. |
NEU1 promote melanoma cells progression in vivo
To determine whether NEU1 plays a role in melanoma cell progression in vivo, we established xenograft tumor models using nude mice. We constructed A875 melanoma cells stably knockdown NEU1 (sh-NEU1) and control (sh-NC) and injected separately the two groups of cells subcutaneously into nude mice. We found that the proliferation rate of NC group was significantly higher than that of NEU1 underexpressed group (Fig. 7a). In addition, the tumor volume from A875 cells after NEU1 underexpressed was significantly lower than that of the control group (Fig. 7a). Moreover, we also tested the effect of NEU1 on melanoma cells metastasis. The nude mice were injected with transfected sh-NEU1 and sh-NC melanoma cells via the tail vein. After 8 weeks of dissection, we found that pulmonary metastatic nodules in the NC group were significantly lower than those in the NEU1 underexpressed group (Fig. 7b). Hematoxylin and eosin (H&E) staining also showed a consistent result, that is, the degree of malignancy was lower, and the metastasis lesion was smaller in the NEU1 underexpressed group. These results showed that NEU1 also significantly promoted the proliferation and metastasis of melanoma cells in vivo.
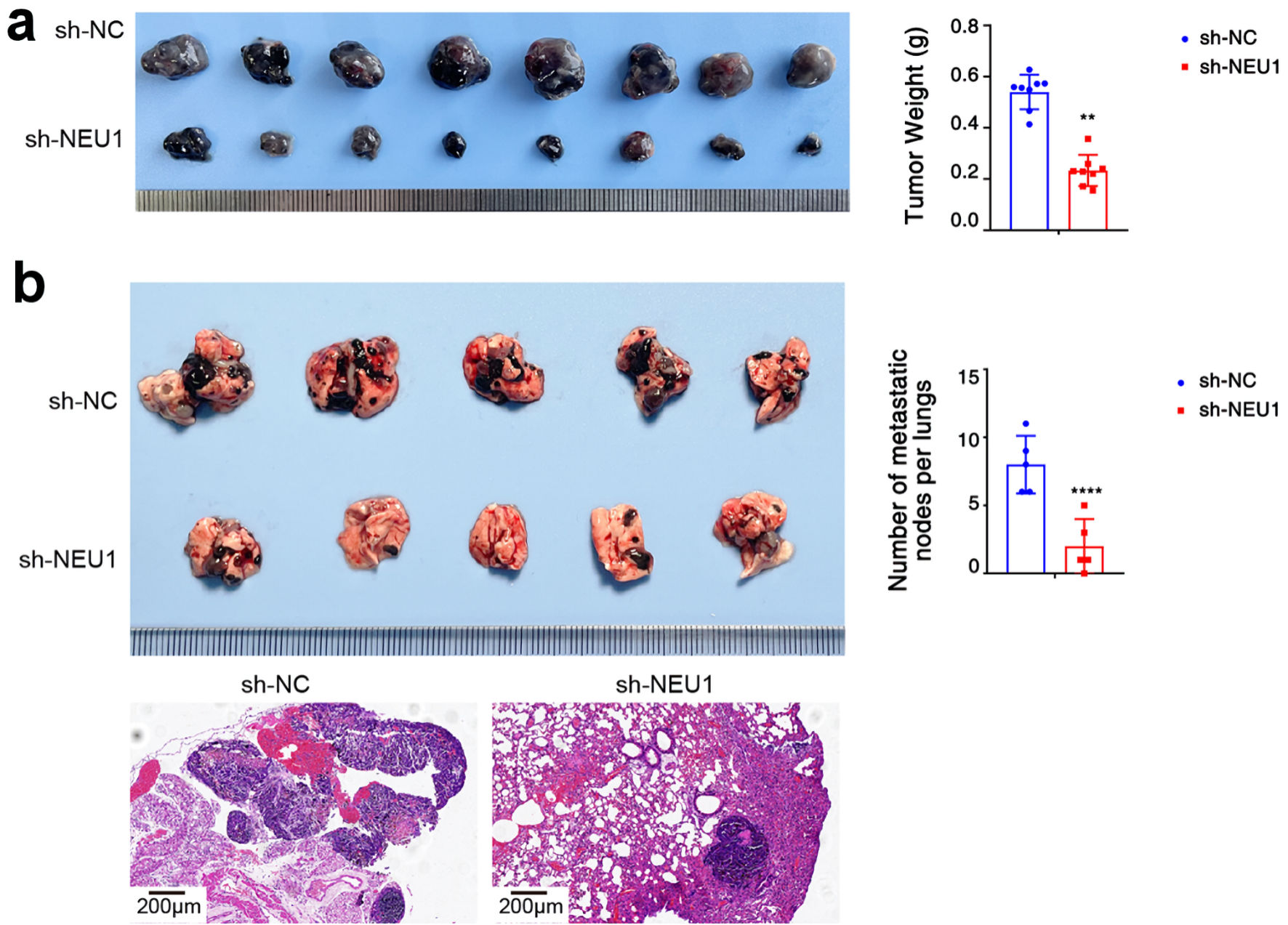 Click for large image | Figure 7. NEU1 promote melanoma cells progression in vivo. (a) Tumor growth of A875 xenografts in nude mice (n = 8 each). Groups 1, 2 mice were injected with NC and sh-NEU1 cells, respectively. (b) After the melanoma cells transfected with sh-NEU1or sh-NC were injected into the tail vein of mice, a representative image of nodules could be seen on the lung surface of mice at 8 weeks (upper panel). Representative images of lung metastasis with H&E staining (lower panel). NEU1: neuraminidase-1. NEU1: neuraminidase-1. |
| Discussion | ▴Top |
Melanoma is a common skin cancer. This type of cancer can spread rapidly in the body. Although surgical resection can effectively treat early primary melanoma, it still has a high recurrence rate. Therefore, new treatment methods including targeted therapy may become a beneficial method for patients with melanoma [33, 34]. In this study, via analyzing public dataset, we identified a novel melanoma-correlated gene NEU1. We found that NEU1 was highly expressed in melanoma, and the higher expression of NEU1, the worse the overall survival rate of melanoma patients. We analyzed the NEU1-related genomic changes and functional networks in melanoma and evaluated their role in tumor immunity through multidimensional analysis. We also demonstrated that NEU1 can promote the proliferation and metastasis of melanoma cells in vivo and in vitro. These results suggest that NEU1 may play a key role as a biomarker for melanoma in tumorigenesis and development.
Sialidase is a kind of glycohydrolases, which can remove sialic acid from the non-reducing ends of various glycoconjugates [35]. Sialidase plays an important role in regulating many physiological processes, such as cell proliferation, apoptosis, and immune monitoring. The changes of sialylation are closely related to malignant tumors. NEU1, NEU2, NEU3 and NEU4 are four sialidase family members that have been confirmed by now. Their localization and substrate specificity are different [36, 37]. NEU1, as a lysosomal neuraminidase, can cleave sialic acid terminal residues from glycoproteins and glycolipids. Although many previous studies reported that NEU1 as an oncogene was upregulated in cancer cells, more and more studies show that the role of NEU1 in different tumors is inconsistent [38]. It is reported that NEU1 can inhibit the progression of human colon cancer in vivo and in vitro [19]. In ovarian cancer, the downregulation of NEU1 expression can effectively inhibit the malignant phenotype of tumor cells [39]. Hou et al also found that NEU1 can promote the growth and metastasis of liver cancer cells [38]. Overexpression of NEU1 in pancreatic cancer can promote tumor growth and metastasis by regulating epidermal growth factor receptor (EGFR) pathway [40]. Zhou et al found that NEU1 can promote cell proliferation and inhibit cell apoptosis by regulating Akt signaling pathway [41]. Our results shows that NEU1 enhance proliferation and invasion of melanoma in vitro and in vivo.
NEU1 regulates downstream molecules through desialylation. Studies have found that integrin β4 is the target of NEU1. Deacetylated integrin β4 will reduce its phosphorylation, thus inhibiting the expression of matrix metalloproteinase (MMP)-7 by regulating FAK and ERK1/2 signaling [19]. Recently, it has been found that noncoding RNA miR-125b can target NEU1 in gastric cancer to regulate the function of gastric cancer cells [42]. In our study, we investigate the potential mechanism underlying NEU1-regulated migration and proliferation of melanoma cells by performing enrichment analysis. Our results show that NEU1-regulated genes are significantly enriched in protein processing, spliceosome and metabolic pathways, which are closely related to tumor characteristics such as cell cycle and angiogenesis [43]. These results indicate that NEU1 may enhance the proliferation and invasion of melanoma by regulating protein processing, splicing and metabolic pathways.
Conclusions
In conclusion, our study confirmed that NEU1 can enhance the proliferation and migration of melanoma cells in vivo and in vitro, indicating that NEU1 may be used as a new prognostic and diagnostic marker of melanoma, thus providing a new target for the treatment of melanoma in the future.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported in part by grants from the National Natural Science Foundation of China (32000665), Shanxi “1331 Project”, Key Research and Development Projects in Shanxi Province (201903D321032), Scientific Research Project of Shanxi Provincial Health Commission (2019142), Applied Basic Research Programs of Science and Technology Commission Foundation of Shanxi Province (201901D211472), and Health Research Project Plan of Shanxi Province (2022119).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conception and design: Qiu Peng, Jin Sheng Wang, Jia Wang. Development of methodology: Liang Gao, Hong Bing Cheng, Qiu Peng, Jia Wang. Acquisition of data: Liang Gao, Hong Bing Cheng, Qiu Peng. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Qiu Peng, Jin Sheng Wang, Jia Wang. Writing manuscript: Qiu Peng, Liang Gao, Jia Wang. Administrative, technical, or material support: Jia Wang. Study supervision: Jin Sheng Wang, Jia Wang.
Data Availability
Cell lines, plasmids and other reagents described in this manuscript are available upon a reasonable request.
| References | ▴Top |
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Bastholt L, Grob JJ, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline - Update 2016. Eur J Cancer. 2016;63:201-217.
doi pubmed - Wen J, Chen W, Zhao B, Xu Q, Liu C, Zhang Q, Xie Z, et al. A carbazole compound, 9-ethyl-9H-carbazole-3-carbaldehyde, plays an antitumor function through reactivation of the p53 pathway in human melanoma cells. Cell Death Dis. 2021;12(6):591.
doi pubmed - Uzdensky AB, Demyanenko SV, Bibov MY. Signal transduction in human cutaneous melanoma and target drugs. Curr Cancer Drug Targets. 2013;13(8):843-866.
doi pubmed - Satzger I, Marks L, Kerick M, Klages S, Berking C, Herbst R, Volker B, et al. Allele frequencies of BRAFV600 mutations in primary melanomas and matched metastases and their relevance for BRAF inhibitor therapy in metastatic melanoma. Oncotarget. 2015;6(35):37895-37905.
doi pubmed - Lei FX, Jin L, Liu XY, Lai F, Yan XG, Farrelly M, Guo ST, et al. RIP1 protects melanoma cells from apoptosis induced by BRAF/MEK inhibitors. Cell Death Dis. 2018;9(6):679.
doi pubmed - Arcidiacono P, Ragonese F, Stabile A, Pistilli A, Kuligina E, Rende M, Bottoni U, et al. Antitumor activity and expression profiles of genes induced by sulforaphane in human melanoma cells. Eur J Nutr. 2018;57(7):2547-2569.
doi pubmed - Ugurel S, Rohmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, Larkin J, et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur J Cancer. 2016;53:125-134.
doi pubmed - Helgadottir H, Rocha Trocoli Drakensjo I, Girnita A. Personalized medicine in malignant melanoma: towards patient tailored treatment. Front Oncol. 2018;8:202.
doi pubmed - Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29(22):3085-3096.
doi pubmed - Jarkowski A, 3rd, Khushalani NI. BRAF and beyond: Tailoring strategies for the individual melanoma patient. J Carcinog. 2014;13:1.
doi pubmed - Pacheco I, Buzea C, Tron V. Towards new therapeutic approaches for malignant melanoma. Expert Rev Mol Med. 2011;13:e33.
doi pubmed - Alon R, Chen S, Puri KD, Finger EB, Springer TA. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J Cell Biol. 1997;138(5):1169-1180.
doi pubmed - Monti E, Preti A, Venerando B, Borsani G. Recent development in mammalian sialidase molecular biology. Neurochem Res. 2002;27(7-8):649-663.
doi pubmed - Dridi L, Seyrantepe V, Fougerat A, Pan X, Bonneil E, Thibault P, Moreau A, et al. Positive regulation of insulin signaling by neuraminidase 1. Diabetes. 2013;62(7):2338-2346.
doi pubmed - Annunziata I, Patterson A, Helton D, Hu H, Moshiach S, Gomero E, Nixon R, et al. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-beta secretion via deregulated lysosomal exocytosis. Nat Commun. 2013;4:2734.
doi pubmed - Amith SR, Jayanth P, Finlay T, Franchuk S, Gilmour A, Abdulkhalek S, Szewczuk MR. Detection of Neu1 sialidase activity in regulating Toll-like receptor activation. J Vis Exp. 2010;43:e2142.
doi pubmed - Abdulkhalek S, Amith SR, Franchuk SL, Jayanth P, Guo M, Finlay T, Gilmour A, et al. Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for Toll-like receptor activation and cellular signaling. J Biol Chem. 2011;286(42):36532-36549.
doi pubmed - Kato T, Wang Y, Yamaguchi K, Milner CM, Shineha R, Satomi S, Miyagi T. Overexpression of lysosomal-type sialidase leads to suppression of metastasis associated with reversion of malignant phenotype in murine B16 melanoma cells. Int J Cancer. 2001;92(6):797-804.
doi pubmed - Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, Kato K, Sakuraba H, et al. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28(9):1218-1229.
doi pubmed - O'Shea LK, Abdulkhalek S, Allison S, Neufeld RJ, Szewczuk MR. Therapeutic targeting of Neu1 sialidase with oseltamivir phosphate (Tamiflu(R)) disables cancer cell survival in human pancreatic cancer with acquired chemoresistance. Onco Targets Ther. 2014;7:117-134.
doi pubmed - Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649-658.
doi pubmed - Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98-W102.
doi pubmed - Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174.
doi pubmed - Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1.
doi - Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401-404.
doi pubmed - Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166-180.
doi pubmed - Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, et al. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11(20):7234-7242.
doi pubmed - Haqq C, Nosrati M, Sudilovsky D, Crothers J, Khodabakhsh D, Pulliam BL, Federman S, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci U S A. 2005;102(17):6092-6097.
doi pubmed - Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Med. 2007;4(5):e176.
doi pubmed - Wang Z, Jensen MA, Zenklusen JC. A practical guide to the cancer genome atlas (TCGA). Methods Mol Biol. 2016;1418:111-141.
doi pubmed - Steven A, Seliger B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care (Basel). 2018;13(1):16-21.
doi pubmed - Fridman WH, Galon J, Dieu-Nosjean MC, Cremer I, Fisson S, Damotte D, Pages F, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344:1-24.
doi pubmed - Garutti M, Targato G, Buriolla S, Palmero L, Minisini AM, Puglisi F. CDK4/6 Inhibitors in Melanoma: A Comprehensive Review. Cells. 2021;10(6):1334.
doi pubmed - Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. 2021;384(23):2229-2240.
doi pubmed - d'Azzo A, Bonten E. Molecular mechanisms of pathogenesis in a glycosphingolipid and a glycoprotein storage disease. Biochem Soc Trans. 2010;38(6):1453-1457.
doi pubmed - Monti E, Bonten E, D'Azzo A, Bresciani R, Venerando B, Borsani G, Schauer R, et al. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem. 2010;64:403-479.
doi - Bonten E, van der Spoel A, Fornerod M, Grosveld G, d'Azzo A. Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev. 1996;10(24):3156-3169.
doi pubmed - Hou G, Liu G, Yang Y, Li Y, Yuan S, Zhao L, Wu M, et al. Neuraminidase 1 (NEU1) promotes proliferation and migration as a diagnostic and prognostic biomarker of hepatocellular carcinoma. Oncotarget. 2016;7(40):64957-64966.
doi pubmed - Ren LR, Zhang LP, Huang SY, Zhu YF, Li WJ, Fang SY, Shen L, et al. Effects of sialidase NEU1 siRNA on proliferation, apoptosis, and invasion in human ovarian cancer. Mol Cell Biochem. 2016;411(1-2):213-219.
doi pubmed - Gilmour AM, Abdulkhalek S, Cheng TS, Alghamdi F, Jayanth P, O'Shea LK, Geen O, et al. A novel epidermal growth factor receptor-signaling platform and its targeted translation in pancreatic cancer. Cell Signal. 2013;25(12):2587-2603.
doi pubmed - Zhou X, Zhai Y, Liu C, Yang G, Guo J, Li G, Sun C, et al. Sialidase NEU1 suppresses progression of human bladder cancer cells by inhibiting fibronectin-integrin alpha5beta1 interaction and Akt signaling pathway. Cell Commun Signal. 2020;18(1):44.
doi pubmed - Chang S, He S, Qiu G, Lu J, Wang J, Liu J, Fan L, et al. MicroRNA-125b promotes invasion and metastasis of gastric cancer by targeting STARD13 and NEU1. Tumour Biol. 2016;37(9):12141-12151.
doi pubmed - Zheng X, Peng Q, Wang L, Zhang X, Huang L, Wang J, Qin Z. Serine/arginine-rich splicing factors: the bridge linking alternative splicing and cancer. Int J Biol Sci. 2020;16(13):2442-2453.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


