| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 4, August 2022, pages 216-221
Prospective Cohort Study of Combination Therapy With Abemaciclib and Hormonal Therapy for Chemotherapy-Treated Patients With Hormone Receptor-Positive Metastatic Breast Cancer
Kana Miyaharaa, Kazutaka Naruib, l, Yukari Uemurac, Akimitsu Yamadad, Kazuhiro Arakie, Fumie Fujisawaf, Takahiro Nakayamag, Takashi Ishikawaa, Naruto Tairah, Yuichiro Kikawai, Tomohiko Aiharaj, Hirofumi Mukaik
aDepartment of Breast Oncology and Surgery, Tokyo Medical University, Tokyo, Japan
bBreast and Thyroid Surgery, Yokohama City University Medical Center, Yokohama, Japan
cDepartment of Clinical Research, National Center for Global Health and Medicine, Tokyo, Japan
dDepartment of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Yokohama, Japan
eDepartment of Medical Oncology, Gunma Prefectural Cancer Center, Gunma, Japan
fDepartment of Medical Oncology, Osaka International Cancer Institute, Osaka, Japan
gDepartment of Breast and Endocrine Surgery, Osaka International Cancer Institute, Osaka, Japan
hDepartment of Breast and Endocrine Surgery, Okayama University Hospital, Okayama, Japan
iDepartment of Breast Surgery, Kansai Medical University, Osaka, Japan
jBreast Center, Aihara Hospital, Osaka, Japan
kDivision of Oncology/Hematology, National Cancer Center Hospital East, Chiba, Japan
lCorresponding Author: Kazutaka Narui, Department of Breast and Thyroid Surgery, Yokohama City University Medical Center, 4-57, Urafune-cho, Minami-ku, Yokohama 232-0024, Japan
Manuscript submitted June 21, 2022, accepted July 25, 2022, published online August 23, 2022
Short title: Abemaciclib for HR+ MBC
doi: https://doi.org/10.14740/wjon1511
| Abstract | ▴Top |
Background: Combination therapy with cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors and hormonal therapy as the first-line and second-line treatments has already been shown to be effective in patients with hormone receptor-positive (HR+) human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (MBC) in clinical trials. On the other hand, in clinical practice, CDK4/6 inhibitors are used not only as first-/second-line but also as later-line hormonal therapies, or for patients receiving prior chemotherapy in metastatic setting. However, the efficacy and safety of combination therapy in these patients remain unclear. In this study, we evaluate the clinical efficacy and safety of combination therapy with abemaciclib and hormonal therapy for chemotherapy-treated patients with HR+ HER2- MBC.
Methods: This multi-institutional prospective cohort study will involve a total of 300 chemotherapy-treated patients with HR+ HER2- MBC. The primary endpoint is progression-free survival (PFS). Secondary endpoints include overall survival, time to treatment failure, response rate, clinical benefit rate, and adverse events. The preplanned subpopulation analysis is the number of chemotherapy regimens for HR+ HER2- MBC (two or less vs. three or more), prior treatment history with CDK4/6 inhibitors other than abemaciclib (presence vs. absence) and menopausal status (pre vs. post). We also planned to determine PFS of the subpopulation treated with abemaciclib as maintenance therapy after chemotherapy.
Discussion: In this multi-institutional prospective cohort study, we evaluate the clinical efficacy and safety of combination therapy with abemaciclib and hormonal therapy for chemotherapy-treated patients with HR+ HER2- MBC. We also evaluate this combination therapy as maintenance therapy in patients who respond to early-line chemotherapy.
Keywords: Abemaciclib; Hormone receptor-positive metastatic breast cancer; Overall survival; Real-world evidence; Prospective cohort study; Chemotherapy-treated
| Introduction | ▴Top |
A hormone receptor-positive (HR+) human epidermal growth factor receptor 2-negative (HER2-) subpopulation accounts for two-thirds of metastatic breast cancer (MBC) in Japan [1]. Because of the rarity in achieving cure in these patients, the main purpose of treatment is to palliate symptoms, extend survival, and improve quality of life.
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors are oral molecular-targeted agents that suppress tumor proliferation through specific inhibition of CDK4 and CDK6. In clinical trials, combination therapy with CDK4/6 inhibitors and hormonal therapy as the first-line and second-line treatments is extremely effective in patients with HR+ HER2- MBC. Phase III clinical trials of CDK4/6 inhibitors, such as palbociclib [2, 3], abemaciclib [4, 5], and ribociclib [6-8], have shown significant prolongation of progression-free survival (PFS) using combination therapy compared with hormonal therapy alone. Moreover, abemaciclib and ribociclib in combination with hormonal therapy have significantly prolonged overall survival (OS) [9-11]. The adverse events (AEs) caused by CDK4/6 inhibitors appear to be within the acceptable range. Thus, combination therapy with CDK4/6 inhibitors and hormonal therapy is recommended by the National Comprehensive Cancer Network (NCCN) guidelines [12] and The Japanese Breast Cancer Society Clinical Practice Guideline for Breast Cancer 2018 [13]. In Japan, palbociclib and abemaciclib were respectively listed on the National Health Insurance drug price list in December 2017 and November 2018. Ribociclib was listed as off label in July 2020 in Japan.
However, the advent of CDK4/6 inhibitors has complicated the treatment of HR+ HER2- MBC. Although the effectiveness of CDK4/6 inhibitors appeared to have been confirmed, treatment with a single hormonal agent is still one of the options, and there remains another clinical question about treatment sequence among hormonal agents, molecular targeted agents, and less toxic chemotherapeutic agents. This may be due to the high cost of molecular targeted agents and the uncertainness of their effectiveness when considering the sequential order.
Furthermore, there is a lack of phase 3 studies that verify the efficacy and safety of these agents with hormonal therapy as third-line or later treatments and for patients treated by chemotherapy. As for administration after chemotherapy, palbociclib in combination with second-line or third-line hormonal therapy has been reported in the PALOMA-3 trial [3], and ribociclib has been verified in combination with first-line hormonal therapy in the MONALEESA-7 trial [8]. Abemaciclib has not yet been verified for patients after chemotherapy in phase3 trials; chemotherapy-treated cases were excluded in the MONARCH-2 trial [4] and the MONARCH-3 trial [5].
Objectives
The objective of this study is to clarify the efficacy and safety of combination therapy with endocrine therapy and abemaciclib in patients who have treatment history with chemotherapeutics for MBC.
Hypotheses
1) Abemaciclib therapy is safe and effective for patients with MBC previously treated with chemotherapeutics.
2) Abemaciclib therapy is an effective maintenance therapy after chemotherapy.
3) Abemaciclib therapy is effective for patients who have been previously treated with other CDK4/6 inhibitors.
| Materials and Methods | ▴Top |
Study design
This research is a multi-institutional prospective cohort study to evaluate the clinical efficacy and safety of combination therapy with abemaciclib and hormonal therapy for chemotherapy-treated patients with MBC. In addition, we designed three pre-planned analyses evaluating the efficacy and safety of combination therapy with abemaciclib and hormonal therapy for chemotherapy-treated patients with MBC. One is to evaluate maintenance therapy after the chemotherapy which was discontinued for reasons other than disease progression. The other evaluates subpopulations stratified by the number of prior chemotherapy regimens (one or two vs. three or more) and, the third, stratified by prior treatment history with CDK4/6 inhibitors other than abemaciclib. The study design flowchart is shown in Figure 1.
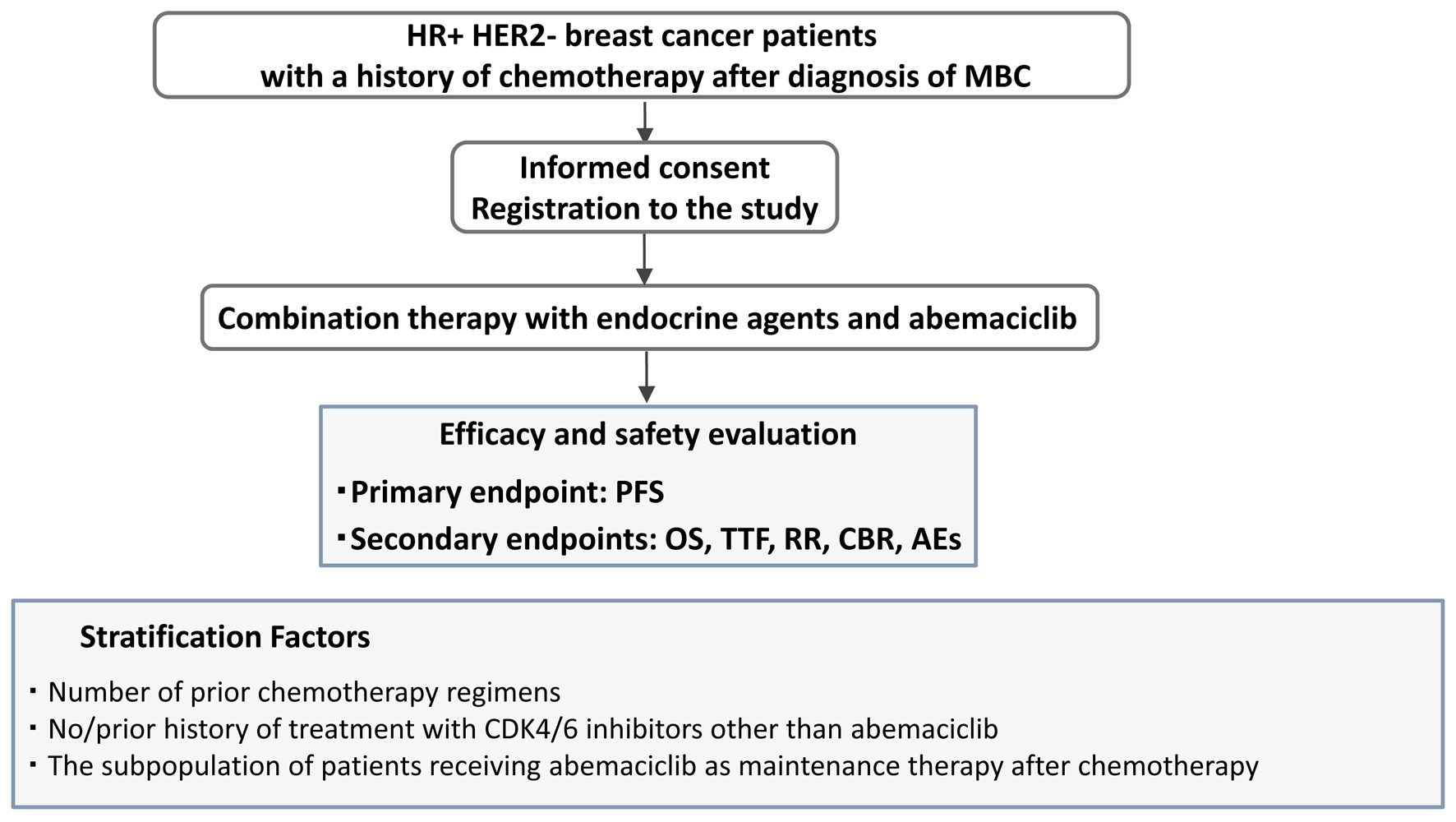 Click for large image | Figure 1. Study design. Patient prospective selection, treatment, and treatment efficacy and safety evaluation using primary and secondary endpoints. |
We will also prospectively investigate drug-induced lung injury caused by abemaciclib as an ancillary study.
Study setting
The study was approved by the Protocol Review Committee of the Comprehensive Support Project for Oncological Research of Breast Cancer (CSPOR-BC). This study was registered at the UMIN Clinical Trials Registry as UMIN 000037395. The study was started in December 2019 and will be completed in November 2022. Patient enrollment will be completed in November 2022 (extended 1 year due to insufficient registration).
Endpoints
The primary endpoint is PFS, defined as the time from the date of registration to disease progression or death from any cause, whichever occurred first. The secondary endpoints are OS (time from registration to death from any cause), time to treatment failure (TTF: time from the enrollment date set as the start date until the day on which progression is confirmed, all-cause death occurs, or treatment is discontinued, whichever comes first), response rate (RR: proportion achieving complete or partial response), clinical benefit rate (CBR: complete response, partial response, or stable disease ≥ 6 months), and AEs. AEs are measured by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Patients
Patients must meet all the following inclusion and exclusion criteria to be eligible as participants in this study.
Inclusion criteria were: 1) women ≥ 20 years old who are diagnosed with “histologically confirmed primary breast cancer”; 2) women whose breast cancer is HR+ HER2-; 3) women who are diagnosed with “difficult to treat” MBC; 4) women with a history of chemotherapy after diagnosis of MBC; 5) women who plan to receive abemaciclib treatment; 6) women who are scheduled to start abemaciclib treatment from 21 days or later after completion of the previous chemotherapy and 14 days or later after completion of radiotherapy; 7) women with no recent history of treatment with CDK4/6 inhibitors; 8) women with no previous history of abemaciclib treatment; 9) woman with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 - 2; 10) women whose vital organ function is normal; and 11) women from whom written informed consent to participate in the study is obtained.
Exclusion criteria were: 1) women for whom endocrine therapy is not indicated; 2) women who require oxygen inhalation because of dyspnea under resting conditions; 3) women who have previous history of gastrectomy or small bowel resection, except for mucosal resection; 4) women who have Crohn’s disease, ulcerative colitis, or chronic diarrhea of grade 2 or higher; 5) women who are pregnant or nursing; 6) women undergoing treatment for bacterial or fungal infection; 7) women who have active hepatitis B or C; 8) women who have serious cardiac disease; and 9) women whose primary care physicians considered that they are unsuitable for inclusion in this study.
Sample size
Since the PALOMA3 trial is the closest approximation to our current study (in terms of containing patients that had received prior chemotherapy), we have used PFS from that study as our benchmark. Hence, the expected median PFS in the current clinical trial is set at 7.7 months.
Meanwhile, in studies investigating efficacy of palbociclib for chemotherapy-treated patients, the median PFS was reported to be 5.9 months in patients treated with one or two regimens, and 4.3 months in patients treated with three regimens or more [14]. As prognosis therefore appears to correlate inversely with number of treatment regimens, we expect a median PFS of 7.7, 5.9, and 4 months in patients treated with one, two, or three or more regimens, respectively.
In this study, it is important to verify that the median PFS is 6 months or longer in patients previously treated with a single regimen chemotherapy. Therefore, we will include a sample size that can be used to determine the median PFS in these patients with high accuracy. If expected median PFS is 8 months under conditions where 160 patients are enrolled within 24 months of the enrollment period and within 36 months of the total study period, the probability that 95% confidence interval (CI) falls into less than 4 months is 80%; the probability rises to 90% if 95% CI falls into less than 4.5 months. We will therefore set the sample size at 300 to ensure that at least 160 patients will have been treated with a single regimen of chemotherapy by the end of this study. Under these conditions, we can accurately determine the median PSF in patients treated with two or more regimens of chemotherapy and in those receiving maintenance therapy.
Discontinuation of subjects
Under the following conditions, participation in the study by subjects from whom consent has been obtained can be discontinued. 1) When subjects withdraw their consent. 2) When the principal investigator decides that withdrawal is appropriate.
Treatment methods
In combination with endocrine agents, abemaciclib (150 mg) is usually administered orally twice daily to an adult. Dosage should be reduced according to the condition of each patient as appropriate. There will be no provision on endocrine agents to be used in combination with abemaciclib. This will be determined through consultation between the attending physician and the patient as part of routine clinical practice.
Data collection
Clinical data are prospectively obtained from the records generated in each institution. In this study, the personnel will enter and correct data using Research Electronic Data Capture (REDCap).
Data management
In the REDCap system, revision records of data correction will be recorded in a log file. Other than for data monitoring, data access for quality management is granted to the director of data management in this study.
Statistical analysis
All patients who receive abemaciclib and who meet the inclusion criteria will be included in the analysis. The median values of PFS, OS, and TTF will be calculated. Survival curves will be calculated using the Kaplan-Meier method. Following this, the 1-, 2-, and 3-year survival rates and corresponding 95% CIs will be calculated. The number and proportion of patients showing response or clinical benefit in the efficacy analysis set will be calculated. The corresponding 95% CIs will also be calculated. Compliance will be calculated for both hormonal therapy and abemaciclib treatment. The highest grade of AEs observed for individual patients will be tallied.
Subpopulation analysis will be conducted to determine whether there is any difference in PFS among subpopulations. The preplanned subpopulation analysis is the number of chemotherapy regimens for HR+ HER2- MBC (two or less vs. three or more), prior treatment history with CDK4/6 inhibitors other than abemaciclib (presence vs. absence) and menopausal status (pre vs. post).
We also planned to determine PFS of the subpopulation treated with abemaciclib as maintenance therapy after chemotherapy. Maintenance therapy in this study is defined as follows: the former chemotherapy was performed more than 9 weeks, the best response of the former chemotherapy was determined as CR, PR or SD, and switching to abemaciclib therapy was planned for a reason other than PD.
Ethics approval
This study has been approved by the Ethical Review Board of Yokohama City University on November 28, 2019 (approval number: B191000019) and registered at the UMIN Clinical Trials Registry as UMIN 000037395. Ethical approval was obtained from all institutes involved in CSPOR-BC. The study will be conducted in accordance with the legal and regulatory requirements as well as the general principles set forth in the International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organizations of Medical Sciences 2002), Guidelines of Good Clinical Practice (International Conference on Harmonization 1996), and the Declaration of Helsinki (World Medical Association 1996 and 2008).
| Discussion | ▴Top |
We started a prospective cohort study to verify the clinical efficacy and safety of combination therapy with abemaciclib and hormone therapy in patients with MBC who underwent chemotherapy based on the hypothesis that this combination therapy may be effective even after chemotherapy. Unlike other CDK4/6 inhibitors, abemaciclib causes neutropenia at a low frequency, thus it could be administered safely and is more acceptable in chemotherapy-treated patients. Additionally, abemaciclib has been reported to have a fairly good outcome in chemotherapy-treated patients with MBC in the MONARCH-1 trial with an objective response rate of 19.7%, a CBR of 42.4%, and a median PFS of 6.0 months [15].
Notably, there are other issues serving as reasons for evaluating abemaciclib combination therapy for chemotherapy-treated patients. One issue is that treatment with a CDK4/6 inhibitor followed by another CDK4/6 inhibitor is not recommended because of the limited data to support it [12]. Basic research has reported the cross resistance of CDK4/6 inhibitors. However, recovery of sensitivity after a temporary cessation of exposure has also been reported [16]. In this regard, it may be better to use a different mechanism after administration of an initial CDK4/6 inhibitor before using another CDK4/6 inhibitor. There is another report on recovery from drug resistance by improving microenvironmental hypoxia and epithelial-mesenchymal transition after the administration of eribulin [17]. Therefore, initial CDK4/6 inhibitor administration followed by chemotherapy then abemaciclib treatment could be one of the options for MBC treatment whose safety and efficacy are worth verifying.
On the other hand, there are some patients requiring more effective treatment from the diagnosis of HR+ HER2- MBC. These patients require chemotherapy from the start of their treatment because of their life-threatening disease or severe symptoms such as pain or discharge from the exposed lesion. Although a favorable response is obtained, it occasionally appears better to discontinue the initial chemotherapy owing to AEs such as cardiotoxicity caused by anthracyclines and peripheral neuropathy caused by taxanes. The combination of CDK4/6 inhibitors and hormone therapy has the potential to be a practical therapy as maintenance therapy after chemotherapy. The clinical efficacy and safety of this combination under such condition will be verified.
In conclusion, this prospective study aims to investigate the efficacy and safety of combination therapy with endocrine therapy and abemaciclib in patients who have treatment history with chemotherapeutics for MBC in daily clinical practice.
Trial status
This study started recruiting patients from December 2019 and will continue recruitment until November 2022 from designated hospitals in Japan. The protocol version is 2.0, which was established on July 8, 2021.
Acknowledgments
We thank Dr. Edward Barroga (http://orcid.org/0000-0002-8920-2607), Medical Editor and Professor of Academic Writing at St. Luke’s International University, Tokyo, Japan for reviewing and editing the article.
Financial Disclosure
The funding for this research is based on the stipulated contract between Eli Lilly Japan K.K. and Comprehensive Support Project for Oncological Research of Breast Cancer. Eli Lilly Japan K.K. is not involved in the decision-making process for any activities.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All eligible patients granted written informed consent.
Author Contributions
All authors conceived and designed. KN and KM conceived, designed, interpreted data, and drafted the manuscript. KA assisted an ancillary study for drug-induced lung injury caused by abemaciclib and paper revisions. YU completed analyses for trial data. HM supervised the overall study and approved the submission of the study design to the journal as the representative of CSPOR-BC. All authors read and approved the manuscript.
Data Availability
Data supporting the trial will be made available on the CSPOR-BC website (https://cspor-bc.or.jp). Detailed datasets for this trial will be kept in the data center of the CSPOR-BC office and will not be accessible during data collection. At trial conclusion, summary data will be made available on the website to aid interpretation and replication of analyses.
Author Note
The authors belong to CSPOR-BC, a clinical trial group studying breast cancer in Japan since 2000. The authors have managed and disseminated the data of various clinical trials from Japan.
| References | ▴Top |
- Kubo M, Kumamaru H, Isozumi U, Miyashita M, Nagahashi M, Kadoya T, Kojima Y, et al. Annual report of the Japanese Breast Cancer Society registry for 2016. Breast Cancer. 2020;27(4):511-518.
doi pubmed - Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936.
doi pubmed - Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439.
doi - Sledge GW, Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, et al. MONARCH 2: Abemaciclib in combination with Fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884.
doi pubmed - Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646.
doi pubmed - Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
doi pubmed - Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465-2472.
doi pubmed - Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, Hurvitz SA, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904-915.
doi - Sledge GW, Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, et al. The Effect of Abemaciclib plus Fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116-124.
doi pubmed - Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, et al. Overall Survival with Ribociclib plus Fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514-524.
doi pubmed - Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, Chow L, et al. Overall survival with Ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307-316.
doi pubmed - Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, et al. Breast cancer, Version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452-478.
doi pubmed - The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer 2018. Tokyo, Japan: Kanehara Shuppan. 2018; p. 95-112.
- Battisti NML, Kingston B, King J, Denton A, Waters S, Sita-Lumsden A, Rehman F, et al. Palbociclib and endocrine therapy in heavily pretreated hormone receptor-positive HER2-negative advanced breast cancer: the UK Compassionate Access Programme experience. Breast Cancer Res Treat. 2019;174(3):731-740.
doi pubmed - Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, Wildiers H, et al. MONARCH 1, A phase II study of Abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218-5224.
doi pubmed - Iida M, Toyosawa D, Nakamura M, Tsuboi K, Tokuda E, Niwa T, Ishida T, et al. Decreased ER dependency after acquired resistance to CDK4/6 inhibitors. Breast Cancer. 2020;27(5):963-972.
doi pubmed - Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, Tohyama O, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014;105(10):1334-1342.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


