| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 13, Number 5, October 2022, pages 311-319
Extramedullary Multiple Myeloma: A Patient-Focused Review of the Pathogenesis of Bone Marrow Escape
Supriya Guptaa, b , Samip Mastera, Christopher Grahama
aDivision of Hematology-Oncology, Feist Weiller Cancer Center, Louisiana State University Health Sciences Center, Shreveport, LA, USA
bCorresponding Author: Supriya Gupta, Division of Hematology-Oncology, Feist Weiller Cancer Center, Louisiana State University Health Sciences Center, Shreveport, LA 71103, USA
Manuscript submitted August 13, 2022, accepted September 22, 2022, published online October 22, 2022
Short title: Extramedullary MM With Liver Involvement
doi: https://doi.org/10.14740/wjon1521
| Abstract | ▴Top |
Multiple myeloma (MM) is a neoplastic clonal proliferation of plasma cells, predominantly in the bone marrow. The presentation of MM in extramedullary tissue, particularly the liver, is uncommon with only a few reported cases in literature. We report a rare and unusual presentation of kappa light chain restricted MM with progression of disease to involve the liver. MM was initially diagnosed on bone marrow biopsy, initially treated with carfilzomib, lenalidomide and dexamethasone, later changed to bortezomib, daratumumab and dexamethasone. There was subsequent progression with a new biopsy-proven myelomatous liver lesion. The patient could not receive high-dose chemotherapy due to multiple co-morbidities and extent of disease and eventually succumbed to her disease rapidly. This article emphasizes the poor prognosis of extramedullary involvement in MM and the pathogenic mechanisms by which it develops. Based on a review of the literature of other cases and case series of solitary or diffuse myeloma involvement in the liver, high-dose chemotherapy in combination with proteasome inhibitors and immunomodulators has the best success rate with less relapse and progressive disease in extramedullary myeloma. Our analysis concluded that the gain of CD44, loss of CD56, loss of very late antigen-4 (VLA-4), imbalance of the chemokine receptor-4-chemokine ligand-12 (CXCR4-CXCL12) axis, metastasis-associated lung adenocarcinoma 1 (MALAT1) upregulation, RAS pathway activation as well as 13q and 17p deletions show an increased propensity of malignant plasma cells to leave the bone marrow and hone in extramedullary sites giving rise to more aggressive extramedullary diseases. Targeted therapeutics such as CD44v-directed therapy and reactivation of p53 to wild-type conformation could potentially be evaluated as treatment options in the future to improve outcomes in this aggressive form of MM, especially in patients with advanced disease and limited treatment options.
Keywords: Multiple myeloma; Extramedullary myeloma; TP53; CD44; CD56; CXCR4; MALAT1
| Introduction | ▴Top |
Multiple myeloma (MM) is a malignant clonal proliferation of plasma cells producing a monoclonal protein predominantly in the bone marrow. This proliferation affects the hematopoiesis in bone marrow, activates osteoclastic bone resorption and produces excessive monoclonal immunoglobulins. In this condition, plasma cells proliferate in the bone marrow and cause extensive bony destruction by osteoclast activation, presenting as pathological fractures as a result of osteopenia and osteolytic bone lesions. The common clinical presentations of MM include anemia (73% of patients), bone pain (58% of patients), elevated serum creatinine (48% of patients), hypercalcemia which may or may not be symptomatic (28% of patients), fatigue and generalized weakness (32% of patients), and weight loss (24% of patients) [1].
The diagnosis of MM is made by observing clonal bone marrow plasma cells ≥ 10% or biopsy-proven bony or soft tissue plasmacytoma plus one of the following: presence of related organ or tissue impairment (CRAB criteria: hypercalcemia - serum calcium > 11 mg/dL, renal failure - creatinine clearance < 40 mL/min or serum creatinine > 2 mg/dL, anemia - hemoglobin < 10 g/dL or > 2 g/dL below normal, and bone lesions - one or more osteolytic lesions ≥ 5 mm in size on skeletal radiography, magnetic resonance imaging (MRI), computed tomography (CT), or positron emission tomography (PET)); presence of a biomarker associated with near inevitable progression to end-organ damage ≥ 60% clonal plasma cells in the bone marrow; involved/uninvolved free light chain (FLC) ratio of 100 or more (provided involved FLC level is at least 100 mg/L); or MRI with more than one focal lesion (involving bone or bone marrow) [2].
Extramedullary myeloma (EMM) is defined as the presence of malignant plasma cell tumors outside the bone marrow which may be from direct spread to the surrounding soft tissue from the bone or arising in extraosseous locations. The initial presentation of extramedullary disease is found typically on advanced imaging techniques: such as CT, MRI, or PET as is the case of our patient. MM with extramedullary, non-osseous disease typically has an incidence of 1-4% in all new presentations and up to 10% on relapse. The incidence of EMM of the bone occurs more frequently at a rate of 7-34%. Myelomatous infiltration of extraosseous tissues is more frequently found in organs rich in reticuloendothelial tissues including liver, spleen and lymph nodes [3]. The most common sites of reported extramedullary involvement of MM are soft tissue surrounding the bone, lymph nodes, liver, spleen, kidneys, lungs, skin, central nervous system, testes, breasts and gastrointestinal tract [4].
This case report is on an elderly female with high-risk kappa light chain disease with relapse involving the liver. It emphasizes the rarity of liver involvement in patients with MM in a relapse setting and progression of disease despite the use of standard agents such as bortezomib, lenalidomide and daratumumab.
| Case Report | ▴Top |
The patient was a 61-year-old Caucasian female who initially presented with neck pain to her primary care physician in August 2017. Her other medical problems included heavy tobacco use (one pack per day for 44 years), type 2 diabetes mellitus, essential hypertension, hyperlipidemia, coronary artery disease (seven drug-eluting coronary stents placed in the past), gastroesophageal reflux disease, anxiety, depression, chronic bursitis of the left hip, and history of placenta previa. A CT scan of the spine showed an osteolytic destructive lesion in the C3 vertebral body which was confirmed on MRI of the spine. She underwent spine stabilization - C3 corpectomy for resection of mass with C2-4 anterior cage resection, fusion, plating in August 2017. The biopsy of the lesion subsequently showed plasmablasts indicating MM. Bone marrow biopsy in August 2017 showed monoclonal plasma cell infiltrate, with 15-20% of marrow cells with kappa light chain restriction compatible with plasma cell neoplasm. She was found to have high-risk disease - 13q deletion, 1q amplification, 14q32/IgH abnormality, and TP53 deletion on fluorescence in situ hybridization (FISH). A PET-CT was done in September 2017 which showed several fludeoxyglucose (FDG) avid osseous lesions in the spine, ribs, bilateral femurs and humeri without evidence of FDG avid extramedullary involvement. The patient was initially started on carfilzomib, lenalidomide and dexamethasone in September 2017 which was later changed after she developed acute kidney injury from the regimen and was switched to bortezomib, daratumumab and dexamethasone in October 2017. The patient was initially showing response to treatment (Table 1). The treatment was completed in January 2018.
 Click to view | Table 1. Staging and Response to Treatment |
PET scan was repeated in January 2018 and showed interval development of several new FDG avid osseous lesions in the axial and appendicular skeleton as well as an incidentally detected intense hypodense lesion inferiorly in the posterior aspect of the right hepatic lobe with another small, but FDG avid focus more superiorly and medially in the posterior aspect of the right hepatic lobe. She was subsequently referred to an academic hospital by her primary oncologist due to progression of disease on PET (Figs. 1-3). Percutaneous biopsy of this newly identified liver lesion was performed from hepatic segment 6 and the pathology showed numerous abnormal plasmacytoid cells consistent with MM (Figs. 4 and 5). Interestingly, the patient did not have any symptoms or evidence of hepatomegaly on examination. Her liver function tests remained normal at the time of detection of the liver plasmacytoma and for the remainder of her disease course.
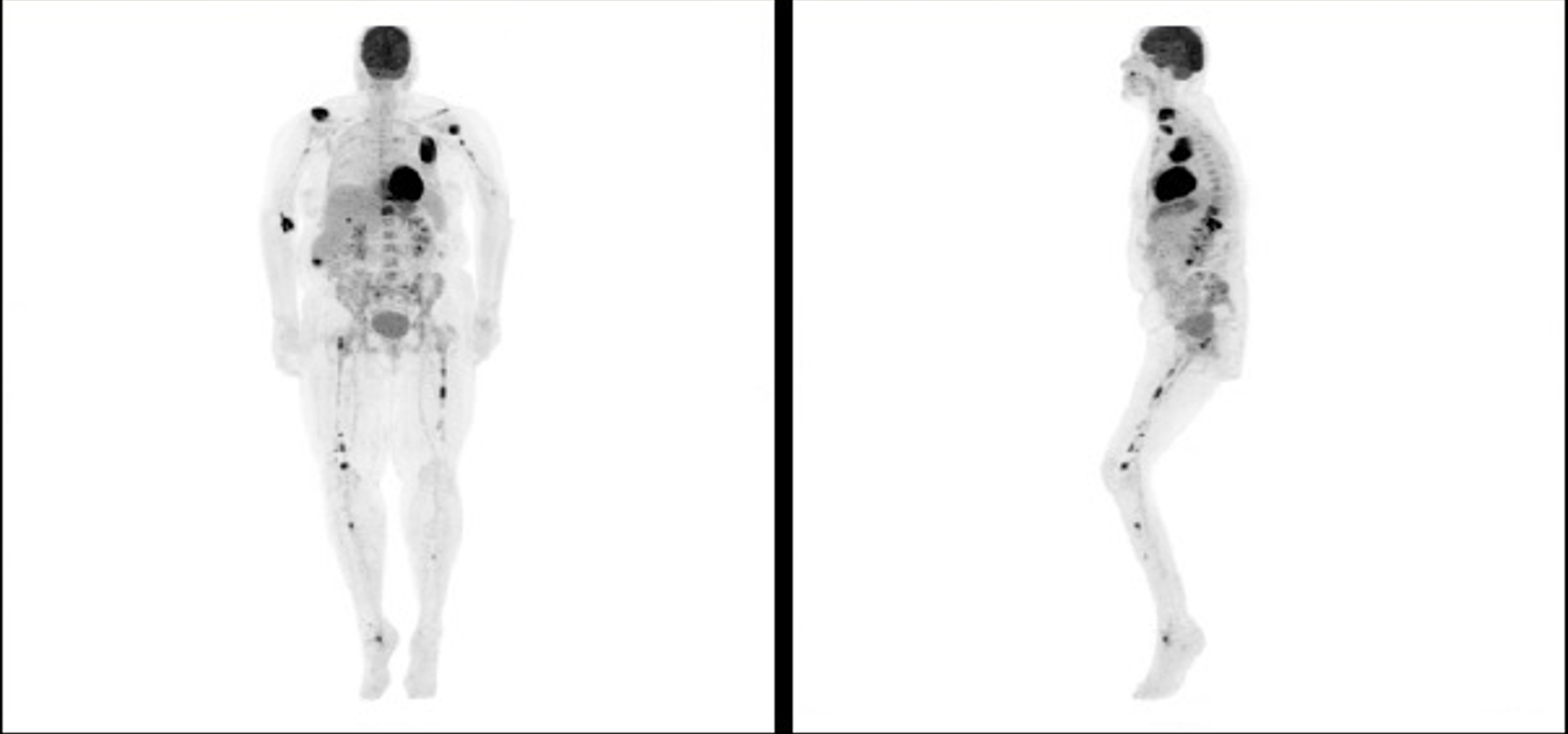 Click for large image | Figure 1. Positron emission tomography scan showing progression of disease following initial chemotherapy. |
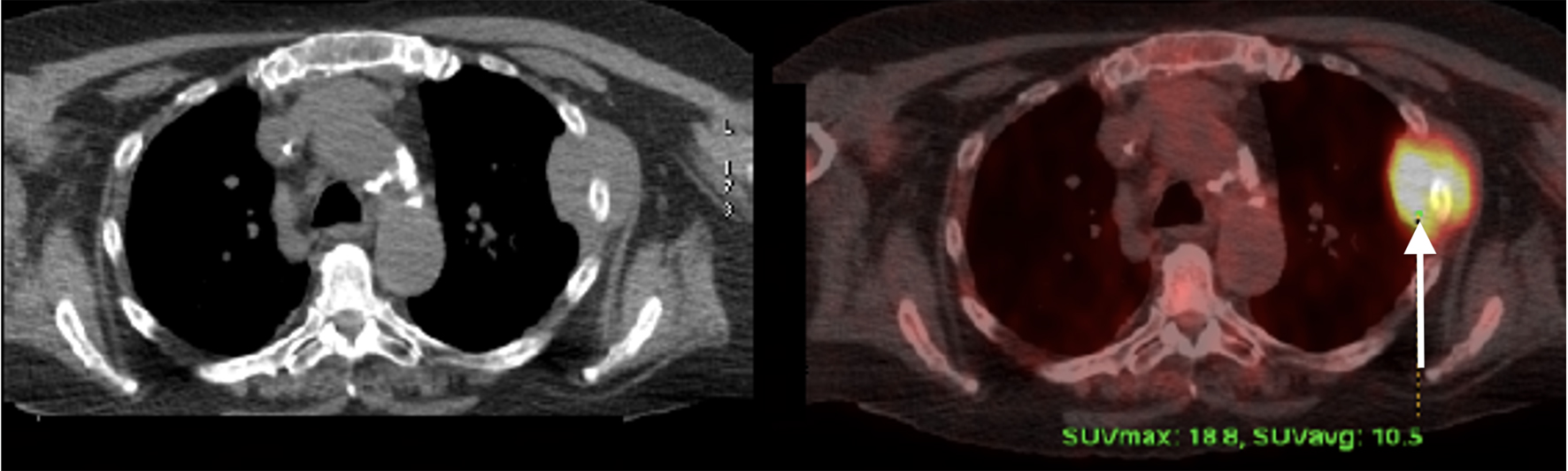 Click for large image | Figure 2. Positron emission tomography scan at the time of relapse showing extraosseous and extramedullary involvement. |
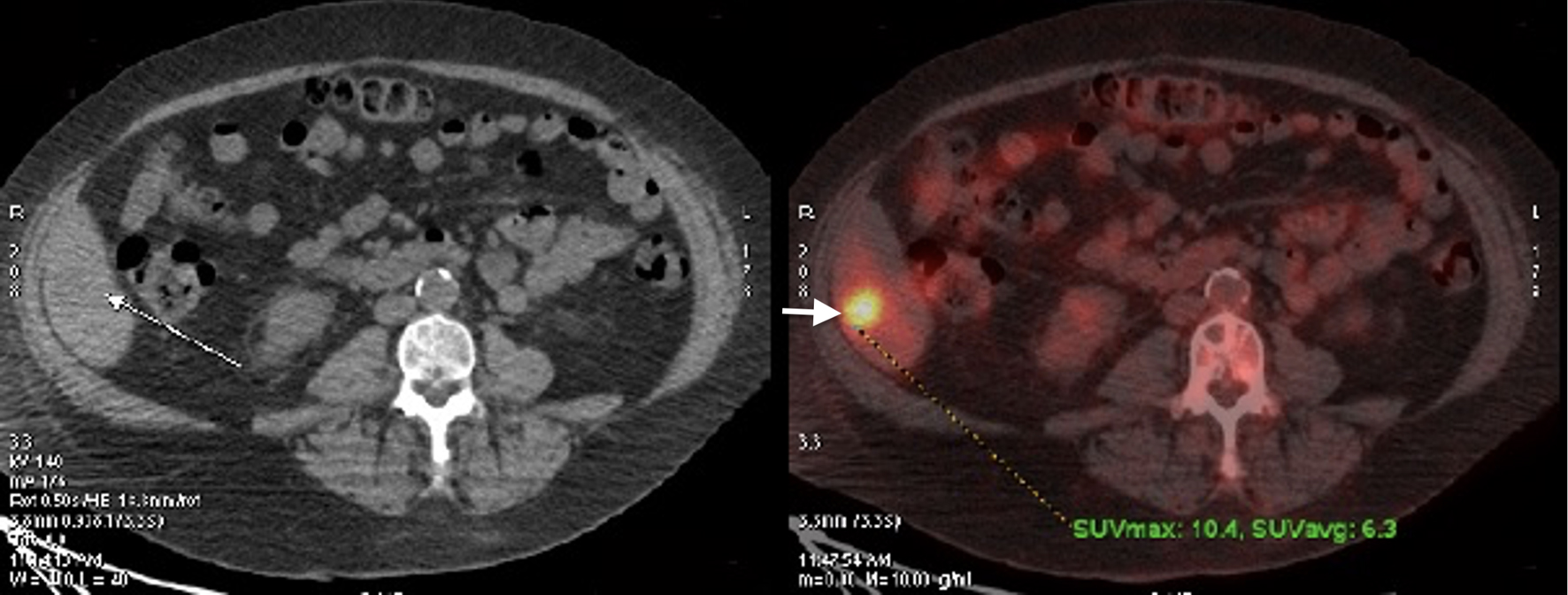 Click for large image | Figure 3. Positron emission tomography scan at the time of relapse showing liver involvement. |
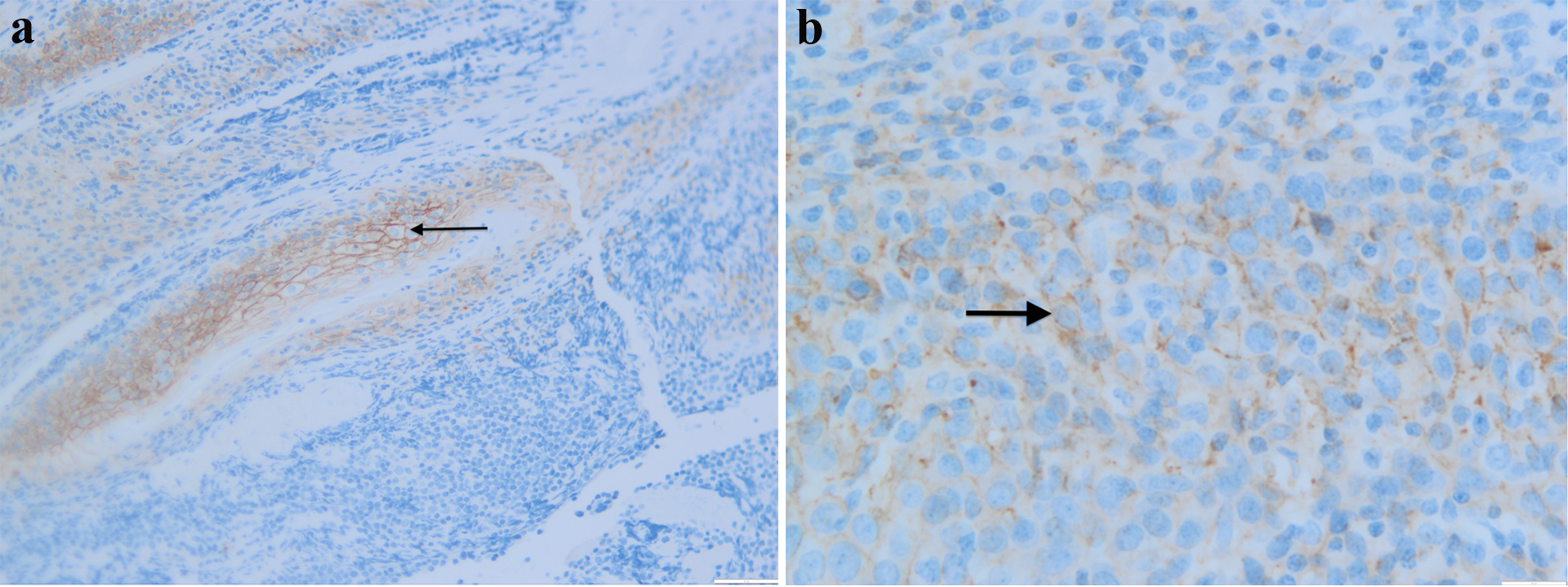 Click for large image | Figure 4. Liver biopsy with CD138 immunostaining showing plasmacytoid cells strongly immunopositive for CD138 at × 30 (a) and × 50 (b) power. |
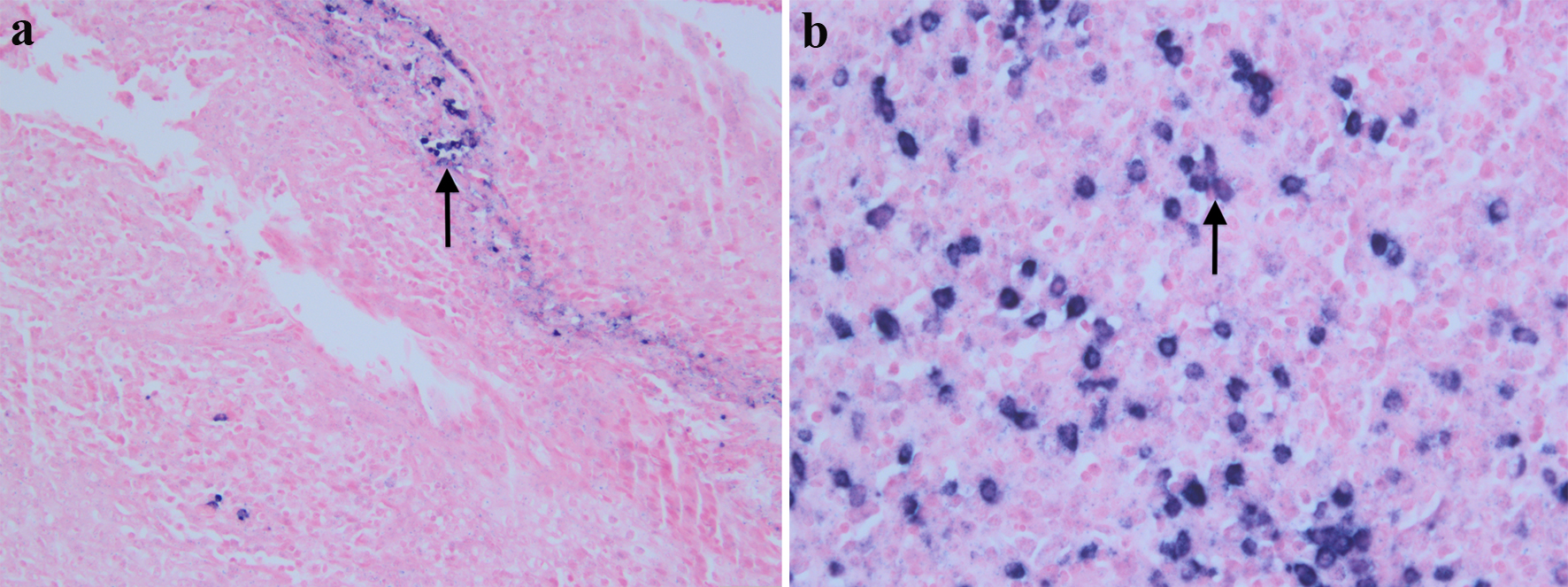 Click for large image | Figure 5. Liver biopsy with kappa in-situ hybridization at × 20 (a) and lambda in-situ hybridization at × 40 (b). The plasmacytoid cells show a kappa restriction. This immuno-histopathological presentation is consistent with kappa-restricted multiple myeloma. |
Due to diffuse aggressive disease and patient’s multiple co-morbidities, she was deemed not to be a candidate for high-dose chemotherapy and bone marrow transplant. An outpatient palliative regimen of cyclophosphamide, pomalidomide and dexamethasone was started in February 2018, and daratumumab was continued. Despite several cycles of treatment, the patient’s disease progressed with rising FLCs. In April 2018, she was started on elotuzumab and lenalidomide and continued on dexamethasone. Within 3 weeks of starting the new therapy, she succumbed to her disease in May 2018 before assessment of the response of the extramedullary disease and liver lesion could be performed.
| Discussion | ▴Top |
The incidence of extraosseous EMM has been shown in several studies. In a study by Varettoni et al involving 1,003 patients with myeloma, the incidence of EMM was 7% at the time of diagnosis and 6% during follow-up [4]. In another case series of 936 patients who were treated for MM, 66 patients presented with extramedullary involvement at diagnosis and 35 patients at the time of disease progression or relapse. According to Usmani et al, liver involvement was seen in 21% of those with extramedullary disease at diagnosis and 34% of patients at the time of relapse or progression [5]. It was concluded that liver involvement was the striking feature in extramedullary disease at disease relapse or progression [4]. Another review of 2,584 patients with MM showed that a mass lesion in the liver or a nodule was detected in only nine patients (0.3%) [6]. The involvement of liver is reported to be the most common site of EMM, comprising of 0.3-3% of all extramedullary diseases, including osseous diseases [5, 6]. Clinical signs of liver involvement are usually hepatomegaly, elevated transaminases, jaundice, ascites and fulminant liver failure. Hepatomegaly is seen in 70% of patients and elevated transaminases in 50-70% [7]. There are two patterns, i.e., diffuse (sinusoidal) and nodular for infiltration that have been described [3]. The diffuse pattern is more common than nodular. A study by Perez-Soler et al described 21 patients with MM where histology showed that diffuse plasma cell infiltration was seen in 10 patients and no cases of nodular infiltration was observed [8]. In another study on 52 autopsied cases of MM, liver involvement was noted in 15 cases (28.8%) with circumscribed nodules in seven cases (13.4%) and diffuse involvement in eight cases (15.4%) [9]. In an analysis by Huang et al [10], four of the 14 cases had liver involvement diagnosed at relapse and 10 on initial diagnosis. Patient characteristics included a mean age of 65 years, presenting with abdominal distension with pain, anorexia, nausea and vomiting. Hepatomegaly was the most common presenting sign, seen in five of 14 patients, followed by jaundice in three out of 14. Twelve patients presented with multiple liver nodules and two with solitary nodules. Most patients (nine out of 14) had kappa-restricted MM (IgG in four cases and IgA in two), while four of 14 had lambda-restricted disease. This was confirmed with histological diagnosis through liver biopsy in 13 of 14 cases. Cytogenetics in one case showed translocation t(11;14). Out of the 14 cases that were evaluated, therapies chosen focused on a melphalan/dexamethasone strategy. Four patients received melphalan and dexamethasone with two patients receiving radiation while the other two did not receive radiations. Three of the patients treated with this strategy initially responded but relapsed, while one patient had stable disease (SD) and one patient who had radiation therapy had improvement. Two patients received the standard VCD regimen (bortezomib, cyclophosphamide and dexamethasone). Both patients had progression and later died with one undergoing trial with lenalidomide before death. One patient receiving bortezomib and dexamethasone and another patient with lenalidomide and dexamethasone had partial response (PR) and complete response (CR), respectively. However, two cases of disease response occurred in patients receiving intensive chemotherapy, one patient receiving vincristine, doxorubicin, dexamethasone and revlimid achieving CR, while the second case receiving carmustine, doxorubicin, melphalan and cyclophosphamide achieving PR. Out of 14 cases, only two patients achieved CR, three had PR, one had SD, five with initial response followed by progressive disease (PD) and three patients died prior to treatment, refused treatment or died or before reassessment of extramedullary disease response. Of note, out of the two patients who had CR, both patients presented with primary, non-relapsed EMM, whereas those with prior-treated MM with relapse with extramedullary disease, had worse outcomes and did not achieve either CR or PR.
Mechanism of EMM
Because of its resistance to multi-regimen chemotherapy and poor prognosis, the exact mechanism of EMM appears to be multifactorial, both in the primary presentation and relapse settings. However, patients who had received treatment prior to extramedullary relapse were not at higher risk of relapse based on the initial agents used. According to Varettoni et al, previous exposure to bortezomib, thalidomide or lenalidomide was not associated with higher risk of extramedullary relapse. Patients who had received treatment prior to extramedullary relapse were not at higher risk of relapse based on the initial agents used [3]. In a retrospective study of 117 MM patients treated on bortezomib-based protocols, all patients who developed EMM lacked translocation t(11;14) (IGH/CCD1) at diagnosis [11]. However, the leading hypothesis is focused on the deletion of p53, loss of CD56, gain of CD44, mutations in the Ras pathway and cell-to-cell adhesion [11].
A study involving a cohort of 663 patients undergoing stem cell transplant for MM evaluated 55 patients with extramedullary disease at diagnosis and at relapse [12]. Eight out of 55 cases had EMM at diagnosis while 42 of the 55 patients developed EMM at time of relapse. The most common sites of EMM at the time of diagnosis were head and neck (31.6%), abdomen (26.3%), chest (21.1%), and central nervous system (12%). Within the abdomen, the most common site of involvement was pancreas, peritoneum, kidney and ileum at the time of diagnosis. The most common location of EMM at the time of relapse was abdomen (40%) and chest (23.9%) with lung (16%) being the most common site, followed by liver (15%). Thirteen patients with EMM had biopsies that were strongly positive for CD44 (92%), chemokine receptor-4 (CXCR4) (38.5%) and CD56 (38.5%). Fourteen patients had abnormal cytogenetics, with the most common cytogenetic mutation being deletion 13q (64%, 9/14). Patients received combination of lenalidomide/bortezomib/dexamethasone (41%) or dexamethasone/thalidomide (45%) or bortezomib/dexamethasone (27%). All patients underwent autologous stem cell transplant (AuSCT) with 15 undergoing allogeneic stem cell transplants (AlSCTs). At a median follow-up time of 8 years, 41 of 55 patients had died with median overall survival (OS) at 4.4 years from diagnosis of MM and 1.3 years from diagnosis of EMM [12]. In a case series of seven patients with EMM, CD56 was absent in all seven out of the seven patients with EMM and CD44 was upregulated [13]. This in conjunction with other alterations in cell-cell adhesion (loss of P-selectin, very late antigen-4 (VLA-4)) of plasma cells to the bone marrow niche (loss of P-selectin, VLA-4) with decreased expression of CCR1 and CC3 chemokine receptors and down-regulation of CXCR4 occurs [11].
CD44 and its effect on tissue homing
CD44, also known as P-glycoprotein 1 or homing cell adhesion molecule (HCAM), has long been associated with metastasis seen in breast and prostate cancer as it directly interacts with the extracellular matrix and allows tumor invasion and is encoded on chromosome 11p13 [11, 14]. Multiple spliced variants with upregulation have been seen in various cancer types such as prostate (CD44v6), colon cancer (CD44v6) and pancreatic cancer (CD44v8). The extracellular membrane component binds to multiple ligands such as hyaluronan, collagen and matrix metalloproteinases. In solid tumors, binding of CD44v to osteopontin allows migration and invasion to distant sites. In hematological malignancies, binding of CD44 to matrix metalloproteinase-9 (MMP-9) allows migration to bone and other soft tissues [14]. Mutations in MMP-9 have been found in chronic lymphocytic leukemia (CLL) cells which allow migration of CLL cells from bone marrow to lymph nodes. This in addition to elevated levels of soluble CD44v has been indicators of advanced disease in CLL. In several studies, upregulation of CD44 has been associated with presence of measurable residual disease (MRD) leading to existence of several subclones causing permanence in bone marrow [15]. This was also found to be the case when biopsies were performed of liver specimens and pleural specimens where plasma cells were found to have high expressions of CD44 [15]. This conveys the idea that CD44 mutations allow diapedesis across the basement membrane, migration to other tissues and the setup of permanence in those tissues. Though CD44 is found ubiquitous on hematopoietic stem cells (HSCs) and epithelial cells limiting a therapy targeting CD44, the CD44v isoform is commonly found in cancer stem cells including adenocarcinomas and hematological malignancies such as MM and acute myelogenous leukemia with low level expression on normal cells [14]. Given this, therapy directed towards the CD44v proves to be a potential worthwhile target. As published by Casucci et al, a CD44v6 directed chimeric antigen receptor T-cell therapy has shown some preclinical response against MM while sparing normal HSCs in mouse models [16]. In this study, 15 samples were taken from patients with varying stages of MM and 13 samples had variable expression of CD44v6. Gene expression was silenced through MM1.S cells by shRNA interference. CD44v6 silencing interfered with ability of MM1.S cells to engraft into the bone marrow of the mice. CD44v6 CAR-T cells recognized circulating monocytes, but not resting T cells, B cells, HSC or endothelial cells, resulting in a monocytopenia that was reversible in vivo with the introduction of an HSV-Mut2 suicide gene that would be turned off by ganciclovir, a retroviral drug used for herpes infection [16]. Human trials are currently in production (NCT04427449).
CXCR4 and its effect on EMM
CXCR4 is associated with plasma cells homing to the bone marrow and loss of this leads to bone marrow escape. The CXCR4-chemokine ligand-12 (CXCL12) axis is important for homing HSC to the bone marrow microenvironment. CXCR4 is the main chemokine found in MM, CLL and acute lymphoblastic leukemia (ALL) cells and plays an important role in trafficking these cells to the bone marrow [15]. CXCL12 is expressed on bone marrow stromal and endothelial cells and binds to CXCR4 found on MM cells [15]. CXCRL12 has several isoforms including isoform alpha which is present on liver endothelial cells. In patients with MM, its expression is found in bone marrow areas with high MM cell infiltration [17]. Inhibition of the CXCL12-CXCR4 axis by plerixafor causes disruption of the bone marrow niche, causing MM cells to circulate in the peripheral blood. As CXCL12 is associated with the hypoxia-induced-factor-alpha (HIF-alpha) pathway, it has been felt that bone marrow hypoxia further drives increased CXCR4 expression to the bone marrow. Increased CXCR4 amounts have been shown to increase acquisition of an epithelial mesenchymal transition allowing escape from the bone marrow niche. Studies with bortezomib showed that treatment with bortezomib reduces the CXCR4 expression, favoring plasma cell escape from the bone marrow niche, which could explain why extramedullary disease is more common at relapse. Furthermore, as mentioned previously, hypoxia upregulated CXCL12 expression in the liver, which might additionally explain egress to the liver from bone marrow [17]. In a study done by Deng et al, it was found that patients with MM with high levels of lactate dehydrogenase (LDH) were also found to have EMM [18]. Bone marrow hypoxia leads to aerobic glycolysis which activates HIF-1-alpha and LDH-A which additionally leads to plasma cells leaving the bone marrow.
VLA-4 mechanism for EMM
VLA-4, also known as integrin α4β1, is an important process for cell-to-cell adhesion as seen between MM cells and the bone marrow niche. It is a noncovalent heterodimer that binds to vascular cell adhesion molecule-1 (VCAM-1) and fibronectin. Activated VLA-4 in MM cells binds with high affinity to bone marrow stromal cells which increases cell adhesion, and increases drug resistance. Ablation in VLA-4 in mouse studies using clustered regularly interspaced short palindromic repeats (CRISPR) technology as shown by Hathi et al demonstrated decreased adhesion to the bone marrow stroma, however showed increased propensity of extramedullary disease [19].
CD56 and effect on EMM
CD56 is known as neural-cell adhesion molecule (NCAM) and is responsible for cell-cell adhesion of neurons and skeletal muscle but also is found on natural killer (NK) cells, and CD4+/CD8+ lymphocytes, monocytes, dendritic cells and to an extent, myelomatous plasma cells [20]. Physiologically, CD56 is a phenotypic activation marker and predictor of normal NK cells, and NK cells were found to be negative in human immunodeficiency virus infections, chronic hepatitis, as well as post stem cell transplant as demonstrated by Van Acker [20]. In the bone marrow, CD56 is found on mesenchymal stromal cells and provides niche for hematopoietic stromal cells. Previously, CD56 was found to be strongly positive in MM with up to 70-80% of patients expressing CD56 [21]. The loss of CD56 has been shown to be a poor prognostic factor in myeloma [11]. A meta-analysis performed by Zhang et al showed that CD56 negative MM had poor OS compared to CD56 positive MM (hazard ratio (HR) = 1.8, P = 0.001) [21]. In regards to myeloma, CD56 is paramount for maintaining connection to the bone marrow niche and holding myeloma cells together, with loss of CD56 in myeloma cells being associated with extramedullary spread [22, 23]. In addition to loss of myeloma cell adhesions, a CD56 low state leads to secretion of MMP-9 causing escape through the basement membrane [21]. However, when AuSCT was performed, CD56 expression had no correlation with prognosis (HR = 1.16, P = 0.583) which might mean that AuSCT abrogated the negative prognostic effect [21].
13q deletion and 17p deletion in EMM
Both chromosome 13 and chromosome 17 are intact for tumor suppression and associated loss has been associated with tumorigenesis. The retinoblastoma (Rb) tumor suppressor gene is located on chromosome 13, while p53 tumor suppressor gene is located on chromosome 17 [24]. Through sentinel mapping of the chr13 gene in MM, 80-90% of patients with this mutation had loss of the entire gene and 10-20% had interstitial deletion involving chromosome band 13q14-q21 located distally to the Rb gene. It has been hypothesized that loss of 13q is considered as a gate-keeper mutation for development of MM as it is seen in up to 40-50% of cases of MM by FISH. It is frequently associated with FGFR mutations (t(4;14)) or t(14;16)(q23;q23) which are known to be poor prognostic indicators in MM. In patients with these mutations, incidence of monosomy 13 or 13q deletions increases to 90%. The deletion is also found in 65% of chromosome 1q mutations. However, in monoclonal gammopathy of unknown significance (MGUS), only 21% carry the -13/13q, suggesting that loss of 13 is a potential key of transformation to MM [24]. In regards to p53 tumor suppressor gene mutations in MM, these events are relatively uncommon with 5-10% occurring in newly diagnosed MM and not seen in MGUS (0/56 patients analyzed at a single institution) [25]. In patients who do have p53 mutations, OS is shorter at 7.9 months compared to 25.7 months and patients are more likely to present with plasma cell leukemia (25%), present at time of relapse, and associated with extramedullary disease. According to a study which looked at 834 patients with MM at a single institution, among those with p53 mutated at presentation, 34% (10 out of 29) with EMM had deletion of p53, whereas only 11% (29 out of 243) of patients with MM had this deletion [18]. As p53 mutations carry a poor prognosis, P53 stabilizers could be an option for patients with mutated TP53. APR-246, a small molecule that stabilizes mutated p53, has shown promise in TP53-mutated acute myeloid leukemia (AML), and the combination of the small molecule p53 reactivation and induction of massive apoptosis or PRIMA-1(Met) with APR-246 showed efficacy in vitro with harvested MM cells in xenografic mouse models with induced apoptosis in myeloma cells without affecting HSC as well as stabilizing p53 to a normal conformation [26]. The combination with dexamethasone increased this antitumor effect.
Ras pathway activation
In a study by Bezieau et al, seven out of 11 patients with EMM had activating Ras mutations: three out of six patients with identical IgH sequencing had positive activating Ras mutations in their extramedullary plasma cells but not intramedullary plasma cells, suggesting a gain of mutation allowing transition to the periphery [27].
Long non-coding RNA (lncRNA) MALAT1
In addition to genes processing active protein, studies have shown that non-coding RNA can interfere with cellular processes such as proliferation, apoptosis and cell motility. The lncRNA frequently includes more than 200 nucleotides and can work as regulatory RNA, with deregulation occurring in tumor carcinogenesis, metastasis, stem cell differentiation and resistance to chemotherapy. MALAT1 is highly expressed in tissues and activates gene expression through polycomb proteins, and is found widespread through cancer metastasis [28]. The non-coding RNA comes from chromosome 11q13 and is upregulated by IgH enhancer as can be seen in t(11;14) myeloma. Because of its association with metastasis in solid tumors, Handa et al looked at MALAT1 expression in 114 myeloma patients through the use of cell lines [28]. EMM was observed in 44 patients at time during disease course, with 39 at time of diagnosis. EMM was found frequently in patients with deletion of 17p (P = 0.009). MALAT1 expression was higher in MM compared to MGUS (P < 0.001) and significantly higher in EMM patients (7.7) compared to MM patients (5.5) (P = 0.05). Interestingly, MALAT1 expression was 10,000-fold higher in EMM cells than bone marrow myeloma cells from the same patients. In intramedullary plasma cells, high expression of MALAT1 had worse OS (mOS of 1.75 years) compared to low MALAT1 group (mOS of 5.03 years). It was shown that proteasome inhibitors and anthracycline-based regimens increased MALAT1 expression in cell lines that were given bortezomib and doxorubicin [28].
Conclusion/learning points
MM is typically known as a disease of bone marrow plasma cells. Extramedullary presentation is usually associated with advanced disease, refractoriness to treatment and early mortality. The mechanism of extramedullary spread is thought to be multifactorial and is poorly understood. Some of the proposed mechanisms of extramedullary spread include gain of CD44, loss of CD56, loss of VLA-4, imbalance of the CXCR4-CXCL12 axis, MALAT1 upregulation, RAS pathway activation as well as 13q and 17p deletions which increase the propensity of malignant plasma cells to leave the bone marrow and hone in extramedullary sites. These mutations and immune-escape phenomenon can lead to bone marrow escape, causing local and widespread disease. Overall, given that the mutations involved in escape include well-known oncogenes such as RAS as well as loss of tumor suppressor p53 and Rb, patients who have EMM have a worse prognosis as shown by our literature review showing poor response to conventional and aggressive chemotherapy. The introduction of anti-myeloma therapies such as the proteosome inhibitor bortezomib ablates the host-myeloma environment allowing bone marrow escape. Whether that is by the downregulation of CXCR4, the inhibition of VLA-4, the upregulation of MALAT1 through cell stress, anti-myeloma therapy disrupts the bone marrow-myeloma cell niche at a potential cost of EMM at time of relapse. More data would need to be collected in response to newer agents for refractory/relapse MM to determine if these agents can reduce morbidity and mortality in patients with EMM.
A potential future target in this disease would be an anti-CD44v antibody or CAR-T cell therapy targeting the plasma cells’ ability to leave the bone marrow and hone to other tissues. Another potential target in TP53-mutated MM would be a small molecule reactivator of the P53 protein to restore it to its wild-type conformation. However, until then, further studies will need to be elucidated to determine if these current therapies or new therapies can decrease the risk of EMM as well as treating relapsed disease.
Testing for CD44 and CD56 is not standard of care at the time of diagnosis or relapse. TP53 and Rb tests are usually performed as a part of FISH. Based on our review, as CD44 and CD56 are predictive of extramedullary spread, poor prognosis and possible target in the future, it may be worthwhile to obtain the tests at the time of diagnosis and relapse. We also recommend changing the adaptive imaging strategies by using CT and PET imaging rather than just skeletal surveys in this at-risk population to detect extraosseous disease at the time of biochemical relapse. Patients with EMM may also benefit from more intensive therapies such as quadruplets instead of doublets or triplets due to its more aggressive disease course; however, this would need to be investigated further.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Conflict of Interest
None to declare.
Informed Consent
Informed consent could not be obtained as patient passed away prior to conceptualization of this project and next-of-kin could not be reached.
Author Contributions
Supriya Gupta contributed to conceptualization, data curation, data interpretation and analysis, reviewing of literature, writing manuscript, data assimilation and formatting, procurement of clinical data and images, submission. Samip Master contributed to conceptualization, supervision, verification of work, critical revision. Christopher Graham contributed to conceptualization, data curation, data interpretation and analysis, reviewing of literature, writing manuscript, procurement and interpretation of clinical images, supervision, verification of work, critical revision and editing of manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
MM: multiple myeloma; EMM: extramedullary myeloma; MRI: magnetic resonance imaging; CT: computed tomography; PET: positron emission tomography; FISH: fluorescence in situ hybridization; PR: partial response; CR: complete response; SD: stable disease; PD: progressive disease; AuSCT: autologous stem cell transplant; AlSCT: allogeneic stem cell transplant; OS: overall survival; MMP-9: matrix metalloproteinase-9; CLL: chronic lymphocytic leukemia; MRD: measurable residual disease; HSC: hematopoietic stem cell; CXCR4: chemokine receptor-4; ALL: acute lymphoblastic leukemia; CXCL12: chemokine ligand-12; LDH: lactate dehydrogenase; VLA-4: very late antigen-4; VCAM-1: vascular cell adhesion molecule-1; CRISPR: clustered regularly interspaced short palindromic repeats; NCAM: neural-cell adhesion molecule; HCAM: homing cell adhesion molecule; MGUS: monoclonal gammopathy of unknown significance; MALAT1: metastasis-associated lung adenocarcinoma 1
| References | ▴Top |
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
doi pubmed - International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749-757.
doi - Thomas FB, Clausen KP, Greenberger NJ. Liver disease in multiple myeloma. Arch Intern Med. 1973;132(2):195-202.
doi pubmed - Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21(2):325-330.
doi pubmed - Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, Alsayed Y, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97(11):1761-1767.
doi pubmed - Wu XN, Zhao XY, Jia JD. Nodular liver lesions involving multiple myeloma: a case report and literature review. World J Gastroenterol. 2009;15(8):1014-1017.
doi pubmed - Yoon YS, Min YH, Chon CY, Park C, Lee SJ, Hahn JS, Ko YW, et al. Liver involvement in multiple myeloma proven by peritoneoscopy—a case report. Yonsei Med J. 1993;34(1):90-97.
doi pubmed - Perez-Soler R, Esteban R, Allende E, Tornos Salomo C, Julia A, Guardia J. Liver involvement in multiple myeloma. Am J Hematol. 1985;20(1):25-29.
doi pubmed - Oshima K, Kanda Y, Nannya Y, Kaneko M, Hamaki T, Suguro M, Yamamoto R, et al. Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am J Hematol. 2001;67(1):1-5.
doi pubmed - Huang H, Bazerbachi F, Mesa H, Gupta P. Asymptomatic multiple myeloma presenting as a nodular hepatic lesion: a case report and review of the literature. Ochsner J. 2015;15(4):457-467.
- Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34(1):1-20.
doi pubmed - Weinstock M, Aljawai Y, Morgan EA, Laubach J, Gannon M, Roccaro AM, Varga C, et al. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br J Haematol. 2015;169(6):851-858.
doi pubmed - Dahl IM, Rasmussen T, Kauric G, Husebekk A. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. Br J Haematol. 2002;116(2):273-277.
doi pubmed - Senbanjo LT, Chellaiah MA. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front Cell Dev Biol. 2017;5:18.
doi pubmed - Redondo-Munoz J, Garcia-Pardo A, Teixido J. Molecular players in hematologic tumor cell trafficking. Front Immunol. 2019;10:156.
doi pubmed - Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122(20):3461-3472.
doi pubmed - Ullah TR. The role of CXCR4 in multiple myeloma: Cells' journey from bone marrow to beyond. J Bone Oncol. 2019;17:100253.
doi pubmed - Deng S, Xu Y, An G, Sui W, Zou D, Zhao Y, Qi J, et al. Features of extramedullary disease of multiple myeloma: high frequency of p53 deletion and poor survival: a retrospective single-center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15(5):286-291.
doi pubmed - Hathi D, Chanswangphuwana C, Cho N, Fontana F, Maji D, Ritchey J, O'Neal J, et al. Ablation of VLA4 in multiple myeloma cells redirects tumor spread and prolongs survival. Sci Rep. 2022;12(1):30.
doi pubmed - Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front Immunol. 2017;8:892.
doi pubmed - Zhang L, Huang Y, Lin Y, Zhang A, Zou R, Xu H, Wang S. Prognostic significance of CD56 expression in patients with multiple myeloma: a meta-analysis. Hematology. 2022;27(1):122-131.
doi pubmed - Ceran F, Falay M, Dagdas S, Ozet G. The Assessment of CD56 and CD117 Expressions at the Time of the Diagnosis in Multiple Myeloma Patients. Turk J Haematol. 2017;34(3):226-232.
doi - Blaheta RA, Beecken WD, Engl T, Jonas D, Oppermann E, Hundemer M, Doerr HW, et al. Human cytomegalovirus infection of tumor cells downregulates NCAM (CD56): a novel mechanism for virus-induced tumor invasiveness. Neoplasia. 2004;6(4):323-331.
doi pubmed - Liebisch P, Dohner H. Cytogenetics and molecular cytogenetics in multiple myeloma. Eur J Cancer. 2006;42(11):1520-1529.
doi pubmed - Herrero AB, Rojas EA, Misiewicz-Krzeminska I, Krzeminski P, Gutierrez NC. Molecular mechanisms of p53 deregulation in cancer: an overview in multiple myeloma. Int J Mol Sci. 2016;17(12):2003.
doi pubmed - Saha MN, Jiang H, Yang Y, Reece D, Chang H. PRIMA-1Met/APR-246 displays high antitumor activity in multiple myeloma by induction of p73 and Noxa. Mol Cancer Ther. 2013;12(11):2331-2341.
doi pubmed - Bezieau S, Devilder MC, Avet-Loiseau H, Mellerin MP, Puthier D, Pennarun E, Rapp MJ, et al. High incidence of N and K-Ras activating mutations in multiple myeloma and primary plasma cell leukemia at diagnosis. Hum Mutat. 2001;18(3):212-224.
doi pubmed - Handa H, Kuroda Y, Kimura K, Masuda Y, Hattori H, Alkebsi L, Matsumoto M, et al. Long non-coding RNA MALAT1 is an inducible stress response gene associated with extramedullary spread and poor prognosis of multiple myeloma. Br J Haematol. 2017;179(3):449-460.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


