| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Short Communication
Volume 13, Number 6, December 2022, pages 403-408
Cerebral Infarction Caused by Trousseau’s Syndrome Associated With Lung Cancer
Shoko Ikutaa, Kanako Nishimatsua, Nao Shoshiharaa, Kentaro Masuhiroa, Seigo Minamia, b
aDepartment of Respiratory Medicine, Osaka Police Hospital, Tennoji-ku, Osaka 543-0035, Japan
bCorresponding Author: Seigo Minami, Department of Respiratory Medicine, Osaka Police Hospital, Tennoji-ku, Osaka 543-0035, Japan
Manuscript submitted September 4, 2022, accepted October 19, 2022, published online December 1, 2022
Short title: Cerebral Infarction Induced by Lung Cancer
doi: https://doi.org/10.14740/wjon1523
| Abstract | ▴Top |
Background: Lung cancer is one of the common cancers that can cause Trousseau’s syndrome. However, there are few reports of cerebral infarction due to Trousseau’s syndrome associated with lung cancer. The aim of this study is to investigate the clinical features of lung cancer-related cerebral infarction and effective management practice.
Methods: Japanese patients diagnosed with Trousseau’s syndrome-related cerebral infarction associated with lung cancer between August 2012 and November 2021 in our hospital were retrospectively enrolled. Clinical data, treatment, and outcomes of the patients were collected.
Results: Ten patients were enrolled. The median age was 65 years (range: 43 - 84 years). All patients had advanced lung cancer. The histological types were adenocarcinoma (n = 8), pleomorphic carcinoma (n = 1), and small cell lung cancer (n = 1). Recurrent cerebral infarction occurred in six patients. Among four patients who had continued heparin since the initial infarction, recurrence occurred in one. D-dimer was high in all 10 patients at the initial cerebral infarction. D-dimer level at the time of recurrent cerebral infarctions was higher than that at the first cerebral infarctions. Since performance status declined in nine patients, one patient continued anticancer drugs after cerebral infarction. Four patients died within 100 days of the onset of cerebral infarction.
Conclusions: Cerebral infarction of lung cancer-related Trousseau’s syndrome has poor prognosis. Heparin may be effective in controlling the condition. In addition, D-dimer may serve as a marker of cancer-related thrombosis.
Keywords: Trousseau’s syndrome; Cerebral infarction; Lung cancer; Heparin; D-dimer; Arterial thromboembolism
| Introduction | ▴Top |
Trousseau’s syndrome is cancer-associated hypercoagulative disorder in the broad definitions, and also means cancer-associated cerebral artery thromboembolisms in the narrow definitions. Thrombotic events are the second leading cause of death in patients with cancer, after death from cancer itself [1]. In particular, cerebral infarction has a significant impact on prognosis because it can cause a decrease in performance status.
Although lung cancer is one of the most common cancer types associated with cerebral infarction [2], clinical characteristics and optimal management of Trousseau’s syndrome-related cerebral infarction in lung cancer are unclear.
Here, we summarized and analyzed the characteristics of 10 lung cancer patients with Trousseau’s syndrome-related cerebral infarction and discussed effective management practice.
| Materials and Methods | ▴Top |
Japanese lung cancer patients diagnosed with lung cancer between August 2012 and November 2021 in our hospital were retrospectively enrolled. The diagnosis of lung cancer was confirmed pathologically. Among those lung cancer patients, we searched for the patients who had experienced cerebral infarction associated with lung cancer-related Trousseau’s syndrome. The diagnosis of cerebral infarction was based on diffusion-weighted brain magnetic resonance imaging (MRI). Patients treated with anti-vascular endothelial growth factor (VEGF) antibodies, which is known as a risk factor of thrombosis, were excluded. Whether the cerebral infarction was caused by lung cancer or other causes, such as atherosclerosis, was determined after consultation with neurologists or neurosurgeons. We collected and analyzed clinical data of baseline clinical characteristics, anticoagulants, introduction of anticancer drugs, and outcomes.
The study was approved by the Institutional Review Board (IRB); and was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
During the 9 years and 3 months, a total of 2,113 patients were diagnosed with lung cancer in our hospital. There were 970 patients with stage 0 - II, 361 with stage III, 685 with stage IV, and 97 with unidentified stage patients. Ten patients were diagnosed with lung cancer-associated cerebral infarction. The incidence of lung cancer-related cerebral infarction was 0.28% in stage III and 1.31% in stage IV. None of the stage 0 - II patients had a lung cancer-related cerebral infarction. The backgrounds of the 10 patients are summarized in Tables 1 and 2. The characteristics, treatment and outcomes of cerebral infarction are shown in Table 3.
 Click to view | Table 1. Patient Backgrounds |
 Click to view | Table 2. Risk Factors of Atherosclerosis |
 Click to view | Table 3. Characteristics, Treatment and Outcomes of Cerebral Infarction |
The patient backgrounds
The median age was 65 years (range: 43 - 84 years). The patients included seven males and three females. All 10 patients had advanced stage of cancer. The most common histological type was adenocarcinoma (n = 8). One patient had pleomorphic carcinoma which partially contained adenocarcinoma-like mucinous cells, and the other had small cell lung cancer (SCLC). Five patients harbored driver mutations, positive epidermal growth factor receptor (EGFR) mutation in four and positive anaplastic lymphoma kinase (ALK) rearrangement in one. Untreated brain metastasis was observed in two. While one underwent whole brain radiation therapy approximately 1 year before the onset of cerebral infarction, brain magnetic resonance angiography (MRA) showed the vessel wall irregularities due to atherosclerosis and no arterial stenosis. Regarding the risk of atherosclerosis, a smoking history, dyslipidemia, hypertension, and diabetes mellitus were present in eight, one, three, and one, respectively. Brain MRA showed atherosclerotic changes in the vessels in two cases.
As for the timing of onset of cerebral infarction, in nine cases, the cerebral infarction occurred when the cancer was not under control. Four patients had cerebral infarction while their lung cancer was resistant to anticancer treatment and progressed. Four patients had cerebral infarctions when they were scheduled to start their first anticancer drug. One patient was diagnosed with lung cancer after the cerebral infarction occurred. One patient had cerebral infarction while the cancer was stable due to anticancer drugs.
Four patients had other thrombotic complications such as deep venous thrombosis (DVT) (n = 2) and renal infarcts (n = 2). While transthoracic echocardiography was performed in eight patients, nonbacterial thrombotic endocarditis (NBTE) or right-left shunt were not identified in any of the patients. Transesophageal echocardiography was not performed on any patient due to their poor general condition.
The characteristics and treatment of cerebral infarction
Multiple and coincidental cerebral infarctions in bilateral multiple vascular territories were the most frequent radiological features shown by brain MRI (n = 9). As representative cases, the images of cases 1and 2 are shown in Figures 1 and 2, respectively. Nine had neurological symptoms at the time of the initial cerebral infarction, while one was asymptomatic.
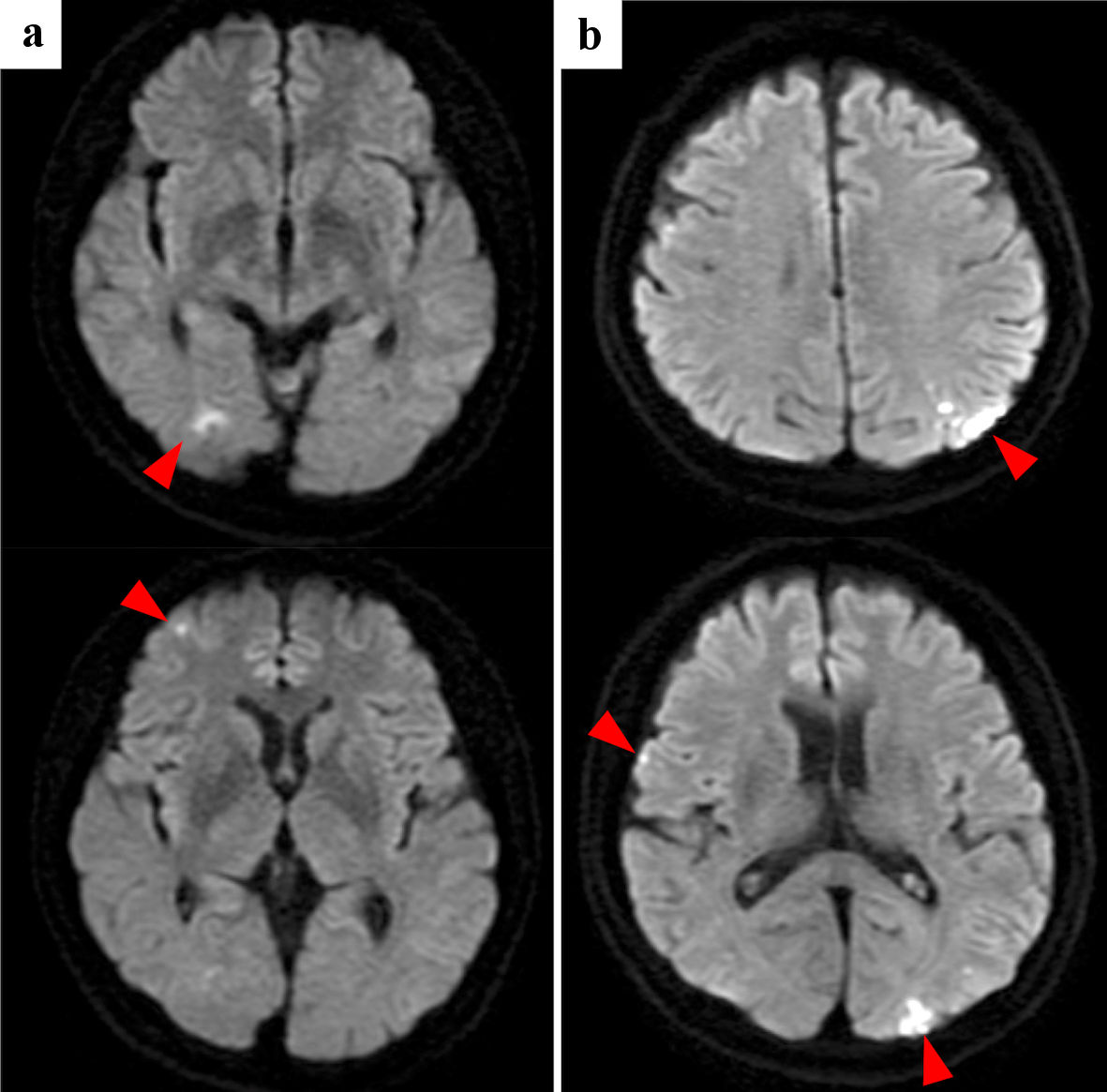 Click for large image | Figure 1. Case 1: brain diffusion-weighted MRI of initial cerebral infarctions (a) and recurrent cerebral infarctions (b). Red arrowheads point cerebral infarctions. MRI: magnetic resonance imaging. |
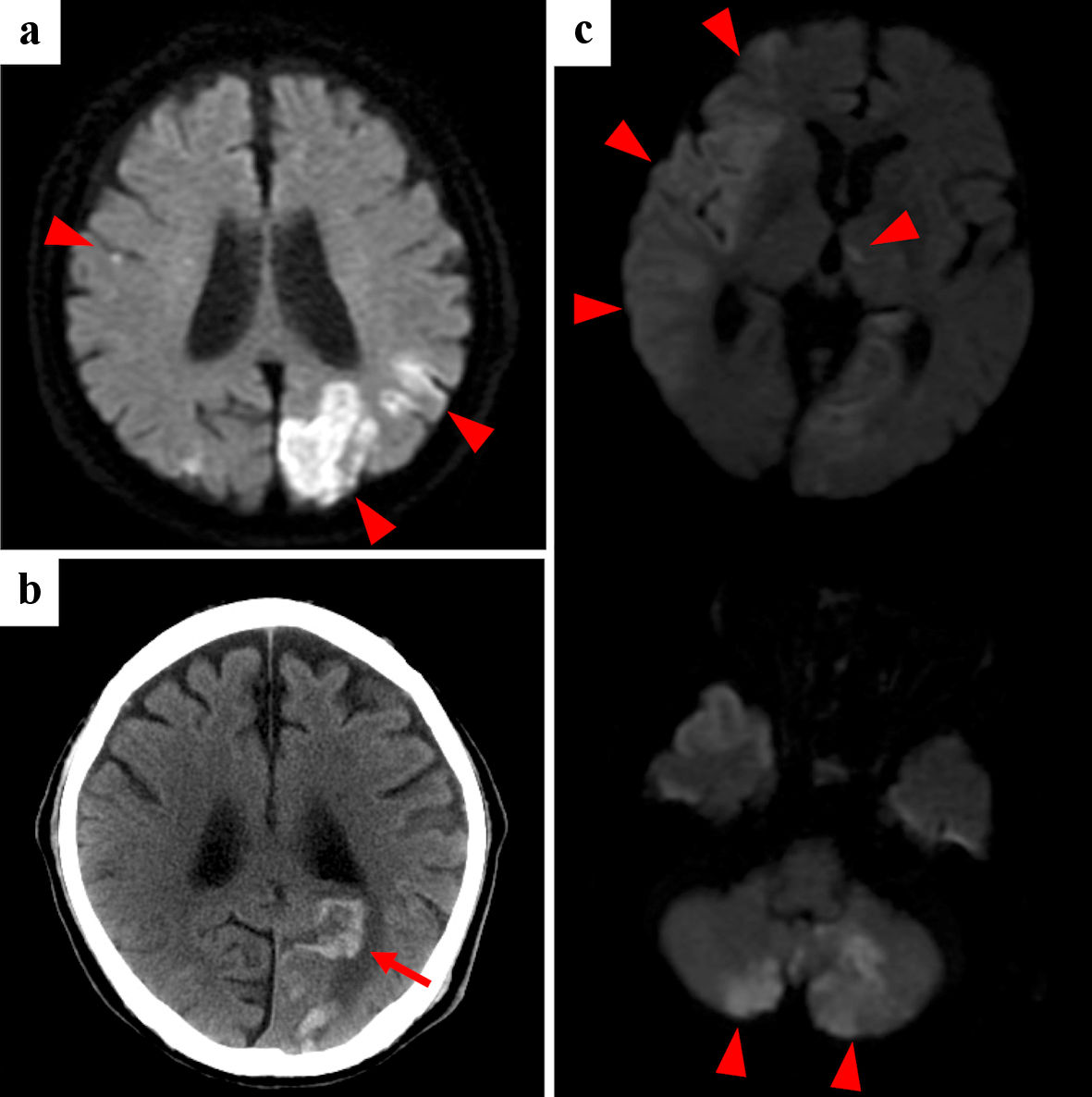 Click for large image | Figure 2. Case 2: brain diffusion-weighted MRI (a, c) and CT (b) of initial cerebral infarctions (a), hemorrhagic infarction (b) and recurrent cerebral infarctions (c). Red arrowheads point cerebral infarctions, and red arrow points hemorrhagic infarction. MRI: magnetic resonance imaging; CT: computed tomography. |
Recurrent cerebral infarctions were observed in six patients, all within 50 days. Five had recurrence despite anticoagulation therapy. Among the four patients who started and continued heparin at the initial onset, one had a recurrence. On the other hand, among the other four patients whose initial treatment was anticoagulants other than heparin or who did not continue heparin, all four had recurrences. Two patients did not receive anticoagulants at the initial onset because of their poor general condition. While heparin was used for initial treatment in four patients, it was used in seven patients during the course of treatment. Moreover, three patients were discharged using self-injection of subcutaneous heparin calcium at home. Since low-molecular-weight heparin has not been approved by Japanese medical insurance for the treatment of cerebral infarction, we used intravenous unfractionated heparin or subcutaneous heparin calcium.
All patients showed high D-dimer levels at the time of both initial and recurrent cerebral infarction. The D-dimer level at the initial onset was 16.1 ± 12.5 µg/mL (mean ± standard deviation (SD)). Among the six patients with recurrent cerebral infarction, five had higher D-dimer at the recurrence compared with the level of the initial onset. In cases 1 and 3, heparin decreased D-dimer within normal range after the recurrences. However, in case 3, heparin was discontinued because of the heparin-induced bleeding.
Outcomes of the patients
Nine patients showed a decrease in performance status (PS) due to cerebral infarction or cancer progression. While the most frequent Eastern Cooperative Oncology Group (ECOG)-PS before cerebral infarction was 1, after cerebral infarction, it became 3. As a result, three patients (cases 1, 2, and 4) received chemotherapy after the cerebral infarction. However, two discontinued the chemotherapy during the first cycle due to a decline in PS caused by recurrent cerebral infarction (cases 2 and 4). Thus, one (case 1) could continue both chemotherapy and long-term heparin calcium self-injection and keep ECOG-PS 1 without recurrent cerebral infarction after the first recurrence. This patient was the only one who survived more than a year. At least, four patients died within 100 days of the onset of cerebral infarction, except for two patients (cases 6 and 10) who were lost to follow-up.
| Discussion | ▴Top |
To our knowledge, this is the first report of a rare condition of cerebral infarction associated with Trousseau’s syndrome due to lung cancer.
First, our study showed that cerebral infarction of Trousseau’s syndrome had seriously poor prognosis. A decline in PS due to cerebral infarction made it difficult to start or continue anticancer drugs. The principle for controlling Trousseau’s syndrome is the treatment of cancer. In this study, cerebral infarction due to Trousseau’s syndrome occurred only in patients with advanced lung cancer which required chemotherapy. Nevertheless, 90% of the patients were unable to continue chemotherapy after cerebral infarction. Cerebral infarctions in Trousseau’s syndrome tended to be multiple and recurrent. Therefore, it was difficult for patients to recover their general condition.
Second, heparin might be effective treatment in preventing recurrence of cerebral infarction. Although there are guidelines recommending low-molecular-weight heparin for the treatment of cancer-related venous thrombosis [3, 4], there is no guideline for cancer-related arterial thrombosis, such as cerebral infarction. However, heparin is the preferred drug in Trousseau’s syndrome, and indeed there were many reports of exacerbation of thrombosis followed by discontinuation of heparin [5, 6]. This is because heparin inhibits a variety of cancer-related thrombogenic pathways, including antithrombin activation, secretin-mediated cancer mucin thrombus production [7], and the tissue factor pathway [8]. On the other hand, most of the direct oral anticoagulants (DOACs) specifically inhibit factor Xa. As Hokusai venous thromboembolism (VTE) cancer study demonstrated the noninferiority of edoxaban to low-molecular-weight heparin [9], DOACs are an available option for the treatment of cancer-related VTE. However, little evidence exists regarding the effects of DOACs on cancer-related arterial thrombosis. In our cases, recurrence of cerebral infarction occurred in four patients with oral antithrombotic drugs such as aspirin and DOACs, but in only one patient with heparin. Among the seven patients who received heparin, six patients had no recurrence.
Third, D-dimer may be a useful marker of Trousseau’s syndrome-related cerebral infarction. Elevated D-dimer in patients with cerebral infarction was reported to suggest a cancer-induced hypercoagulative state [10]. In all of our cases, D-dimer was elevated at the initial cerebral infarction. Moreover, in the six patients with recurrent cerebral infarction, D-dimer levels were still high at the time of recurrence. Ito et al reported that high D-dimer levels in the subacute phase of cerebral infarction associated with Trousseau’s syndrome were related to poor prognosis [11]. Thus, D-dimer monitoring may be useful in assessing whether cancer-associated thrombosis is under control.
This study had some limitations. First, this study lacked statistical analysis due to the small number of cases. Second, this study was a retrospective analysis and might contain selection bias. We are afraid of missing cases of cerebral infarctions in severe conditions and indistinguishable cases from multiple brain metastases. Thus, the incidence rate of this condition may be higher than that of our report.
In conclusion, cerebral infarction of lung cancer-related Trousseau’s syndrome has poor prognosis. Heparin may be effective in controlling the condition. In addition, D-dimer may serve as a marker of cancer-related thrombosis.
Acknowledgments
The authors are grateful to Shoichi Ihara, Shinji Futami, Yu Futami, Taro Koba, Yoshimi Noda, and Saori Amiya at the Department of Respiratory Medicine, Osaka Police Hospital for their medical records, diagnosis, treatment and care of their patients.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The Osaka Police Hospital Ethics Committee approved the study protocol and waiver of the written informed consents from each patient, considering the retrospective design and anonymous clinical data.
Author Contributions
SI designed, collected the data, and drafted the manuscript. All authors critically revised the report, commented on drafts of the manuscript, and approved the final version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490-493.
doi pubmed - Selvik HA, Thomassen L, Bjerkreim AT, Naess H. Cancer-associated stroke: the Bergen NORSTROKE study. Cerebrovasc Dis Extra. 2015;5(3):107-113.
doi pubmed - Watson HG, Keeling DM, Laffan M, Tait RC, Makris M, British Committee for Standards in H. Guideline on aspects of cancer-related venous thrombosis. Br J Haematol. 2015;170(5):640-648.
doi pubmed - Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654-656.
doi pubmed - Alderman CP, McClure AF, Jersmann HP, Scott SD. Continuous subcutaneous heparin infusion for treatment of Trousseau's syndrome. Ann Pharmacother. 1995;29(7-8):710-713.
doi pubmed - Bell WR, Starksen NF, Tong S, Porterfield JK. Trousseau's syndrome. Devastating coagulopathy in the absence of heparin. Am J Med. 1985;79(4):423-430.
doi - Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112(6):853-862.
doi pubmed - Sandset PM, Bendz B, Hansen JB. Physiological function of tissue factor pathway inhibitor and interaction with heparins. Haemostasis. 2000;30(Suppl 2):48-56.
doi pubmed - Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624.
doi pubmed - Kono T, Ohtsuki T, Hosomi N, Takeda I, Aoki S, Sueda Y, Ishihara K, et al. Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int. 2012;12(3):468-474.
doi pubmed - Ito S, Kikuchi K, Ueda A, Nagao R, Maeda T, Murate K, Shima S, et al. Changes in serial D-dimer levels predict the prognoses of Trousseau's syndrome patients. Front Neurol. 2018;9:528.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


