| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 13, Number 5, October 2022, pages 244-248
Giant Retroperitoneal Liposarcoma: Correlation Between Size and Risk for Recurrence
Rozan Marjiyeh-Awwada, Subhi Mansoura, Safi Khuria, b, c
aDepartment of General Surgery, Rambam Health Care Campus, Haifa, Israel
bHPB and Surgical Oncology Unit, Rambam Health Care Campus, Haifa, Israel
cCorresponding Author: Safi Khuri, General Surgery Department,Rambam Health Care Campus, Haa’leya Hashniya, Haifa 31096, Israel
Manuscript submitted September 20, 2022, accepted October 3, 2022, published online October 22, 2022
Short title: Giant Retroperitoneal Liposarcoma
doi: https://doi.org/10.14740/wjon1528
| Abstract | ▴Top |
Soft tissue sarcomas (STSs) are rare tumors that represent almost 1% of adult malignant tumors. The annual incidence rate for such tumors is 2 - 5/100,000 population. The most common type of STS in adults is liposarcoma, which represents 15-20% of adult STSs. It is of mesodermic origin derived from adipose tissues, and known as the most common primary malignant tumor of the retroperitoneum. Other sites of involvement include the extremities, trunk and to a lesser extent the pleural cavity, esophagus, mediastinum and others. Due to the potential large retroperitoneal space, retroperitoneal liposarcoma (RPL) is usually asymptomatic during the initial phase, developing symptoms at a late stage due to large mass compressing nearby retroperitoneal structures. The average diameter and weight of RPL during diagnosis is 20 - 25 cm and 15 - 20 kg, respectively. Several factors were labelled as risk factors for recurrence, such as histological type, tumor grade, age, resectability and tumor size. Controversy exists regarding the relationship between tumor size and recurrence rate, thus, tumor size as a risk factor for recurrence should be clarified. Although there is no consensus regarding the precise definition of giant RPL, it is defined by several literatures as an RPL of greater than 30 cm in diameter or with weight of more than 20 kg. The main purpose of this article is to review the current English literature regarding giant RPL and examine the relationship between tumor size and risk for recurrence.
Keywords: Giant retroperitoneal liposarcoma; Tumor size; Recurrence risk
| Introduction | ▴Top |
Soft tissue sarcomas (STSs) are uncommon tumors that represent approximately 1% of adult malignancies [1]. The reported incidence rate is 2 - 5/100,000 population. According to the fourth edition of the World Health Organization (WHO), there are more than 100 subtypes of soft tissue tumors, the majority of which are STSs. Each subtype of these tumors has a unique clinical, prognostic and therapeutic behavior [2]. The most common site of involvement of STSs is the extremities (41%), followed by the trunk (13%), retroperitoneum (7%), gastrointestinal tract (7%), head and neck (5%), and the uterus (4%). Liposarcoma (12%), leiomyosarcoma (12%) and undifferentiated (pleomorphic) sarcoma (11%) are the most common types of STSs [3].
Liposarcoma is the most common type of STSs in adults and represent about 20% of adult malignant mesenchymal tumors [4]. It is a tumor of mesodermic origin derived from adipose tissues. Although it can affect any part of the body, it usually develops in the extremities, retroperitoneum, trunk and to a lesser extent in the mediastinum, pleural cavity, esophagus, uterus, spermatic cord and others [5-9]. At the retroperitoneal cavity, retroperitoneal liposarcoma (RPL) is the most common primary tumor and represents 40% of all retroperitoneal sarcoma tumors. According to the 2020 edition of the WHO, four types of liposarcoma are recognized: atypical lipomatous tumor/well-differentiated liposarcoma, dedifferentiated liposarcoma, myxoid/round cell liposarcoma and pleomorphic type [10]. The anatomic distribution of liposarcoma subtypes depends on the histologic type; while well-differentiated and dedifferentiated subtypes are more common in the retroperitoneal cavity, pleomorphic and myxoid subtypes are more common in the extremities.
Due to the large potential space in the retroperitoneal cavity, primary RPL can grow to a very large size without causing symptoms. The average diameter of the tumor at diagnosis is 20 - 25 cm with a weight of 15 - 20 kg [11]. Due to the previously mentioned parameters, patients with primary RPL develop symptoms at later stages of the disease, mainly due to mass effect on adjacent organs, and less commonly, by organ invasion [12]. Primary RPL is characterized usually by low rates of complete surgical resection and high rates of tumor recurrence following resection, due to late diagnosis [13].
Giant liposarcoma is defined, by several authors, as a tumor weight of more than 20 kg or tumor diameter of more than 30 cm [14] (Fig. 1).
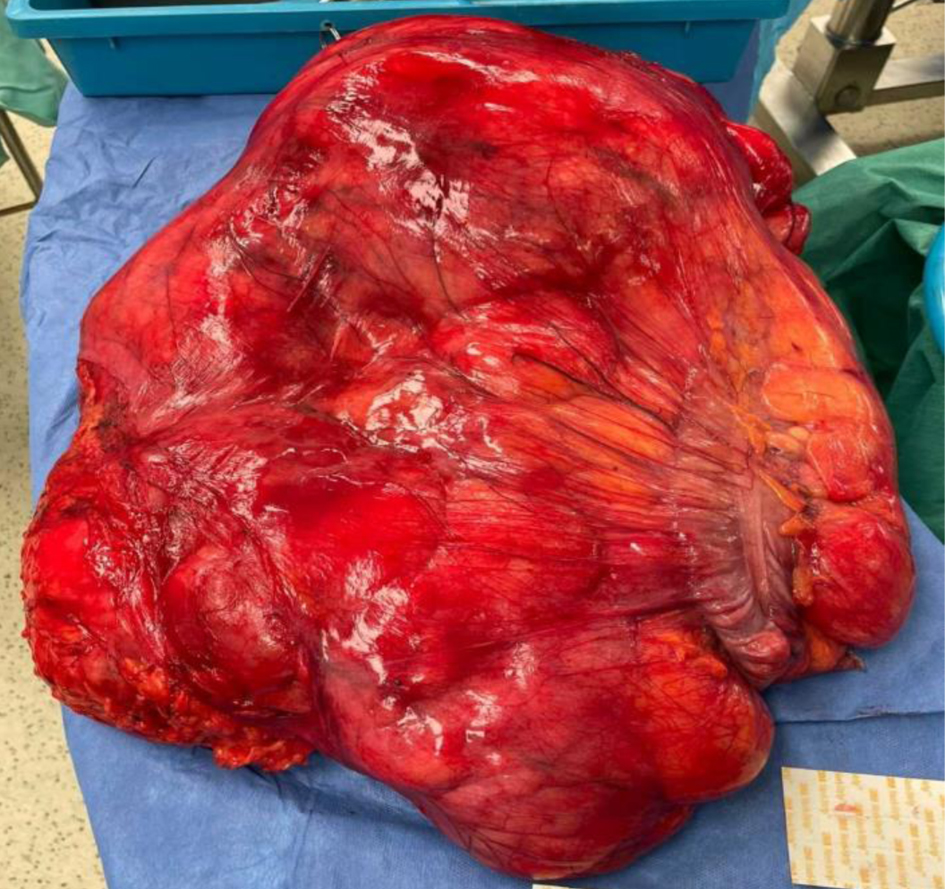 Click for large image | Figure 1. A giant retroperitoneal liposarcoma (diameter: 50 cm; weight: 29 kg). |
The mainstay management for primary RPL is complete surgical resection with negative microscopic margins (R0), when feasible [15]. Even when complete surgical resection is possible, local recurrence rate is high at 66% and 5-year overall survival rate is 54% [16, 17]. Several prognostic factors for recurrence and overall survival for patients with primary RPL have been reported and include age, tumor grade, histological subtype, complete resectability and tumor size, with the latter being a scientific dilemma in the absence of studies in the English literature that examine the relationship between size of the primary tumor and risk for recurrence.
Due to the lack of reported studies about the aforementioned relationship, the aim of this study is to review the pertinent and available studies in the English literature, specifically giant RPL, to figure out if size of the primary tumor is to be considered as a prognostic risk factor for tumor recurrence.
| Methods | ▴Top |
A search in PubMed was conducted, based on the “PICOS” acronym. Headings and text words were used to identify studies (retrospective, prospective, case report and case series) published regarding giant primary RPL.
The following search terms were included: “retroperitoneal liposarcoma”, “liposarcoma of the retroperitoneum”, “giant liposarcoma”, “giant retroperitoneal tumors”, “retroperitoneal tumors”, and “retroperitoneal sarcoma”.
All reported cases of giant primary RPL were included, and data regarding patients’ demographics, tumor size, tumor resectability status, histological type, neo-adjuvant/adjuvant radiation therapy and recurrence were collected.
| Results | ▴Top |
Reviewing the current English literature revealed 24 reported cases of giant primary RPL solely [13, 18-24, 25-31]; 14 case reports and one retrospective study including 10 cases of retroperitoneal dedifferentiated liposarcoma [31]. Of the 24 patients, 14 were males and 10 were females. The average age at diagnosis was 57 years old (age range 40 - 76 years old). The most common presenting symptom (not available in the retrospective study) was increased abdominal girth (or abdominal distension) reported by all patients. Other less reported symptoms were dyspnea, constipation, weight loss, leg edema, weight gain and dyspepsia (Table 1). In the retrospective study by Bachmann et al [31], only dedifferentiated RPLs were included and there were no data regarding clinical presentation, imaging techniques used and specific data regarding tumor size to build up a correlation between size and tumor recurrence. Thus, it was excluded from this review.
 Click to view | Table 1. Demographic Features for Patients With Giant Retroperitoneal Liposarcoma |
The largest tumor diameter was 65 cm, reported by Zeng et al [28], while the smallest one was 30 cm, represented by Zhang et al [13]. The average tumor diameter for all cases was 43.5 cm. Abdominal computed tomography (CT) scan was the most commonly used radiological exam, done for all patients, while abdominal ultrasound (US) and abdominal magnetic resonance imaging (MRI) were used as additional imaging tests for two patients each. Pre-operative diagnosis by a proven biopsy of liposarcoma was available in four patients only [18, 20, 21, 24], and the majority (nine patients) underwent upfront surgical resection without biopsy. All patients were operated on with negative resection margins (R0 resection) achieved in all, except one [19], who had microscopic positive margins (R1 resection). No cases of R2 resection (macroscopic positive margins) were documented. Most operations (eight patients) included resection of the primary tumor along with organs involved, of which the kidney (right or left, depends on the location of the primary tumor) was the organ most commonly resected. The most common histological liposarcoma subtype was well-differentiated liposarcoma (6/14 patients,) followed by dedifferentiated (four cases), myxoid and mixed type (myxoid and pleomorphic - two cases each). Only one patient [18] was treated with adjuvant therapy by means of radiotherapy. Follow-up was not reported for two patients [18, 26]. The average mean of follow-up (by months) for the remaining cases was 20 months (ranges between 3 and 63 months) (Table 2). The majority of patients (9/12) which were followed had no evidence of local or remote recurrence, and only three patients suffered from local tumor recurrence. The histological types for these cases were mixed type liposarcoma, myxoid and well-differentiated tumor [13, 22, 27]. Worth mentioning, when the correlation between the size of the primary tumor and risk for tumor recurrence was examined, we have noticed that it is nonexistent as the largest seven giant tumors (diameter 42 - 65 cm) have no evidence of recurrence at a follow-up of 35 months. On the other hand, the smallest giant tumor (30 cm in diameter) developed very early tumor recurrence at 3 months of follow-up (even though surgical margins were negative at the final histopathological report). As already mentioned before, histological subtypes for the reported cases with recurrence were mainly of the mixed and myxoid types, which can explain the recurrence risk, rather than the tumor size, as these subtypes are known as more aggressive tumors.
 Click to view | Table 2. Tumor Characteristics for the Different Reported Cases |
| Discussion | ▴Top |
Since the introduction of STSs as highly malignant tumors with different types, several prognostic factors, independent of tumor histological type, for local/remote recurrence and overall survival were investigated in a thorough manner by multiple studies (retrospective/prospective). These prognostic factors are diverse and mainly include tumor grade, tumor histology, age, respectability status and tumor size [32]. While several studies had already proved the association between prognostic factors and risk of recurrence, up till now, there is no single study that compares tumor size with tumor recurrence risk. Primary RPL recurrence usually develops within 0.5 - 2 years following surgical resection [33], with rates up to 60% at 5 years follow-up [34].
In the present study, we have reviewed the relevant articles regarding giant primary RPL to investigate the association between initial tumor size and risk for recurrence. Following the statement that tumor size is a risk factor for tumor recurrence, we have decided to review the specific group of patients with giant RPL, as they have a very high risk for recurrence.
As had been shown, in the majority of cases, tumor size was not a risk factor for recurrence. Larger tumors did not recur following R0 surgical resection, while smaller tumors did, as early as 3 months following resection. Tumor subtype (myxoid/mixed) and whether or not contiguous organs had been resected were risk factors for tumor recurrence.
In his study, Sun et al [35] demonstrated that tumor size was not an independent prognostic factor for RPL. In another retrospective study [32], RPL tumors were divided according to tumor grade into two groups: low grade (G1) and high grade (G2-G3). Tumor sizes for both groups were almost identical with median diameter of 27 and 28 cm, respectively for both groups. There was no statistically significant difference between the two groups in terms of first, second or third recurrence during follow-up. Overall survival was significantly worse for patients with high grade tumor than low grade tumors. Singer et al [34] have demonstrated that tumor histology type, tumor grade and contiguous organ resection were significantly associated with tumor recurrence, while tumor size was not an independent risk factor. A retrospective study by Chen et al [36], including 51 patients with primary RPL, showed that tumor size was not an independent risk factor for recurrence or prognosis on univariate and multivariate analysis. The findings of the previous studies including our review exclude tumor size as a prognostic factor for tumor recurrence. Hence, the hypothesis that suggests tumor size of primary RPL is a risk factor for recurrence could be appealed, and further future studies should investigate this claim.
As this specific type of primary RPL is very uncommon, and prospective studies are not available, data regarding management and outcomes are very limited. As is the management of any type of STSs, giant primary RPL should be treated by a multidisciplinary team (MDT) of physicians. Few surgeons and radiation oncologist have gained much experience in treating such patients and thus, patients occasionally receive suboptimal treatment with unsatisfactory surgical and oncological outcomes, especially if treated by unexperienced physicians. According to the NICE recommendation [37] for the management of STSs, which have led to the formation of 15 specialized centers, an MDT must include experienced surgeons, radiologist and clinical oncologists specialized in these tumors. Patients should be referred to centers treating this specific pathological disease.
| Conclusion | ▴Top |
Tumor size has been regarded as one of the risk factors for tumor recurrence, yet studies investigating this hypothesis are lacking in the English literature. Literature findings exclude tumor size as a risk factor for tumor recurrence; hence, the previously mentioned claim should be re-examined, and further future studies are encouraged.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to declare.
Author Contributions
SM designed the research. RM collected and analyzed the data. SK wrote and approved the final paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
STSs: soft tissue sarcomas; RPL: retroperitoneal liposarcoma
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
doi pubmed - Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, eds. WHO classification of tumours of soft tissue and bone. 4th ed. IARC WHO classification of tumours. Vol. 5. WHO Press; 2013.
- Blay JY, Honore C, Stoeckle E, Meeus P, Jafari M, Gouin F, Anract P, et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol. 2019;30(7):1143-1153.
doi pubmed - Mack TM. Sarcomas and other malignancies of soft tissue, retroperitoneum, peritoneum, pleura, heart, mediastinum, and spleen. Cancer. 1995;75(1 Suppl):211-244.
doi - Yang YS, Bai CY, Li ZC, Li WJ, Li Y. Giant primary liposarcoma of the anterior mediastinum: A case report. Medicine (Baltimore). 2018;97(42):e12873.
doi pubmed - Pusiol T, Piscioli I, Rondoni V, Scialpi M. Intrathoracic liposarcoma: Case report with emphasis to histogenesis and site of origin classification problems. Lung India. 2018;35(2):186-187.
doi pubmed - Horitani H, Takezawa K, Fukuhara S, Fujita K, Kiuchi H, Uemura M, Imamura R, et al. [A Case of Spermatic Cord Liposarcoma Difficult to Distinguish from Inguinal Hernia]. Hinyokika Kiyo. 2019;65(9):389-392.
- Liu TT, Wu P, Zhang SD. [A case of giant primary liposarcoma of esophagus]. Zhonghua Zhong Liu Za Zhi. 2018;40(1):62-63.
- Kiuchi K, Hasegawa K, Ochiai S, Kosaka N, Kuroda H, Kaji Y, Fukasawa I. Liposarcoma of the uterine corpus: A case report and literature review. Gynecol Oncol Rep. 2018;26:78-81.
doi pubmed - van de Rijn M, Fletcher JA. Genetics of soft tissue tumors. Annu Rev Pathol. 2006;1:435-466.
doi pubmed - Echenique-Elizondo M, Amondarain-Arratibel JA. [Giant retroperitoneal liposarcoma]. Cir Esp. 2005;77(5):293-295.
doi - Joshi RM, Gangurde GK, Talathi NP, Telavane PP, Singh R, Hanamshetti SR, Adhikari DR. Large retroperitoneal liposarcoma - a series of five cases. Indian J Surg. 2013;75(Suppl 1):64-68.
doi pubmed - Zhang WD, Liu DR, Que RS, Zhou CB, Zhan CN, Zhao JG, Chen LI. Management of retroperitoneal liposarcoma: A case report and review of the literature. Oncol Lett. 2015;10(1):405-409.
doi pubmed - Makni A, Triki A, Fetirich F, Ksantini R, Chebbi F, Jouini M, Kacem M, et al. Giant retroperitoneal liposarcoma. Report of 5 cases. Ann Ital Chir. 2012;83(2):161-166.
- Schwarzbach MH, Hohenberger P. Current concepts in the management of retroperitoneal soft tissue sarcoma. Recent Results Cancer Res. 2009;179:301-319.
doi pubmed - Raut CP, Pisters PW. Retroperitoneal sarcomas: combined-modality treatment approaches. J Surg Oncol. 2006;94(1):81-87.
doi pubmed - Anaya DA, Lahat G, Liu J, Xing Y, Cormier JN, Pisters PW, Lev DC, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. 2009;249(1):137-142.
doi pubmed - Yol S, Tavli S, Tavli L, Belviranli M, Yosunkaya A. Retroperitoneal and scrotal giant liposarcoma: report of a case. Surg Today. 1998;28(3):339-342.
doi pubmed - McCallum OJ, Burke JJ, 2nd, Childs AJ, Ferro A, Gallup DG. Retroperitoneal liposarcoma weighing over one hundred pounds with review of the literature. Gynecol Oncol. 2006;103(3):1152-1154.
doi pubmed - Clar H, Leithner A, Gruber G, Werkgartner G, Beham A, Windhager R. Interdisciplinary resection of a giant retroperitoneal liposarcoma of 25 kg. ANZ J Surg. 2009;79(12):957.
doi pubmed - Hashimoto Y, Hatakeyama S, Tachiwada T, Yoneyama T, Koie T, Kamimura N, Yanagisawa T, et al. Surgical treatment of a giant liposarcoma in a Japanese man. Adv Urol. 2010;2010:943073.
doi pubmed - Bansal VK, Misra MC, Sharma A, Chabbra A, Murmu LR. Giant retroperitoneal liposarcoma- renal salvage by autotransplantation. Indian J Surg. 2013;75(2):159-161.
doi pubmed - De Nardi P, Bissolati M, Cristallo M, Staudacher C. Recurrent giant liposarcoma of the spermatic cord. Urology. 2012;79(1):113-114.
doi pubmed - Sharma M, Mannan R, Bhasin TS, Manjari M, Punj R. Giant inflammatory variant of well differentiated liposarcoma: a case report of a rare entity. J Clin Diagn Res. 2013;7(8):1720-1721.
doi pubmed - Caizzone A, Saladino E, Fleres F, Paviglianiti C, Iaropoli F, Mazzeo C, Cucinotta E, et al. Giant retroperitoneal liposarcoma: case report and review of the literature. Int J Surg Case Rep. 2015;9:23-26.
doi pubmed - Hazen B, Cocieru A. Giant Retroperitoneal Sarcoma. J Gastrointest Surg. 2017;21(3):602-603.
doi pubmed - Oh SD, Oh SJ, Suh BJ, Shin JY, Oh CK, Park JK, Kim YM, et al. A giant retroperitoneal liposarcoma encasing the entire left kidney and adherent to adjacent structures: a case report. Case Rep Oncol. 2016;9(2):368-372.
doi pubmed - Zeng X, Liu W, Wu X, Gao J, Zhang P, Shuai X, Tao K. Clinicopathological characteristics and experience in the treatment of giant retroperitoneal liposarcoma: A case report and review of the literature. Cancer Biol Ther. 2017;18(9):660-665.
doi pubmed - Herzberg J, Niehaus K, Holl-Ulrich K, Honarpisheh H, Guraya SY, Strate T. Giant retroperitoneal liposarcoma: A case report and literature review. J Taibah Univ Med Sci. 2019;14(5):466-471.
doi pubmed - Xu C, Ma Z, Zhang H, Yu J, Chen S. Giant retroperitoneal liposarcoma with a maximum diameter of 37 cm: a case report and review of literature. Ann Transl Med. 2020;8(19):1248.
doi pubmed - Bachmann R, Eckert F, Gelfert D, Strohaker J, Beltzer C, Ladurner R. Perioperative strategy and outcome in giant retroperitoneal dedifferentiated liposarcoma-results of a retrospective cohort study. World J Surg Oncol. 2020;18(1):296.
doi pubmed - Volkov AY, Nered SN, Kozlov NA, Stilidi IS, Archery PP, Antonova EY, Privezentsev SA. [Active surgical approach for retroperitoneal liposarcoma]. Khirurgiia (Mosk). 2021;11:5-11.
doi pubmed - Gupta AK, Cohan RH, Francis IR, Sondak VK, Korobkin M. CT of recurrent retroperitoneal sarcomas. AJR Am J Roentgenol. 2000;174(4):1025-1030.
doi pubmed - Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238(3):358-370; discussion 370-351.
doi pubmed - Sun P, Ma R, Liu G, Wang L, Chang H, Li Y. Pathological prognostic factors of retroperitoneal liposarcoma: comprehensive clinicopathological analysis of 124 cases. Ann Transl Med. 2021;9(7):574.
doi pubmed - Chen J, Hang Y, Gao Q, Huang X. Surgical diagnosis and treatment of primary retroperitoneal liposarcoma. Front Surg. 2021;8:672669.
doi pubmed - Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res. 2016;6:20.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


