| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 6, December 2022, pages 379-386
Advanced Stage Is a Risk for Severe Neutropenia in Breast Cancer Patients Undergoing Neoadjuvant Adriamycin/Cyclophosphamide/Docetaxel Chemotherapy
Kazuki Moroa, f, Masayuki Nagahashia, b, Haruka Uchidaa, Maiko Ojia, Junko Tsuchidaa, Kumiko Yamauraa, Chie Toshikawaa, Mae Nakanoa, Mayuko Ikarashia, Yusuke Muneokaa, Yosuke Tajimaa, Hiroshi Ichikawaa, Yoshifumi Shimadaa, Jun Sakataa, Yu Koyamaa, c, Kazuaki Takabea, d, e, Toshifumi Wakaia
aDivision of Digestive and General Surgery, Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan
bDepartment of Breast and Endocrine Surgery, Hyogo Medical University, Hyogo, Japan
cDepartment of Nursing, Graduate School of Health Sciences, Niigata University, Niigata, Japan
dDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
eDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY, USA
fCorresponding Author: Kazuki Moro, Division of Digestive and General Surgery, Niigata University Graduate School of Medical and Dental Sciences, Chuo-ku, Niigata City, Niigata 951-8510, Japan
Manuscript submitted October 3, 2022, accepted November 15, 2022, published online December 1, 2022
Short title: Advanced Stage Is a Risk for Neutropenia
doi: https://doi.org/10.14740/wjon1530
| Abstract | ▴Top |
Background: Severe neutropenia, including febrile neutropenia, is a major toxicity of systemic chemotherapy that leads to delays in treatment, higher costs, and mortality. Severe neutropenia may occur during neoadjuvant chemotherapy even when the patients are free from known risk factors. Pegfilgrastim, a covalent conjugant of filgrastim that stimulate the production of neutrophils, is used for prevention. The current study aimed to reveal the characteristics of patients who need pegfilgrastim for primary prophylaxis to prevent severe neutropenia, including febrile neutropenia and grade 3 neutropenia, during neoadjuvant chemotherapy.
Methods: A retrospective analysis of 83 patients treated with neoadjuvant adriamycin/cyclophosphamide followed by docetaxel chemotherapy was performed. The factors which associated with severe neutropenia were examined by univariate and multivariate analyses.
Results: Severe neutropenia developed in one of 22 patients (5%) with pegfilgrastim for primary prophylaxis and in 17 of 61 patients (28%) without it. In 83 patients, the incidence of severe neutropenia was significantly decreased in the patients with pegfilgrastim for primary prophylaxis shown by the univariate analysis (P = 0.023) and multivariate analysis (P = 0.030). In 61 patients without pegfilgrastim for primary prophylaxis, the univariate analysis showed that severe neutropenia was associated with tumor size (P = 0.004), clinical stage (P = 0.009), and cancer antigen 15-3 (CA15-3) (P = 0.026). The multivariate analysis showed that clinical stage was associated with severe neutropenia (P = 0.021).
Conclusions: The current study demonstrated that advanced stage is a risk for severe neutropenia in patients treated with neoadjuvant adriamycin/cyclophosphamide followed by docetaxel chemotherapy. Given that prophylaxis with pegfilgrastim was associated with significantly lower incidence of severe neutropenia, patient with advance stage breast cancer may benefit from pegfilgrastim during neoadjuvant chemotherapy.
Keywords: Advanced stage; Breast cancer; Neoadjuvant chemotherapy; Neutropenia
| Introduction | ▴Top |
Neoadjuvant chemotherapy (NAC) had been developed as a systematic treatment before a definitive operation for the treatment of locally advanced or inoperable breast cancer [1]. In addition to its impact on surgery, the role of NAC has expanded to monitor the individual drug response which could predict the risk of recurrence, especially in triple-negative breast cancer and human epidermal growth factor receptor 2 (HER-2)-positive breast cancers [1, 2]. Severe neutropenia, including febrile neutropenia (FN), is one of the major toxicities during systemic chemotherapy, resulting in the delay of treatment and hospitalization, higher costs, and increased mortality [3-5]. Clinical practice guidelines recommend prophylaxis with granulocyte colony-stimulating factor, such as pegfilgrastim, for patients with a higher risk for FN during chemotherapy [6-8]. Many previous studies on chemotherapy for breast cancer demonstrated that older age and a low pretreatment absolute neutrophil count were associated with FN [9, 10]. However, patients without these factors sometimes develop severe neutropenia during NAC.
Adriamycin/cyclophosphamide (AC) followed by docetaxel (DTX) chemotherapy is widely used as regimens of neoadjuvant and adjuvant chemotherapy for breast cancer [11]. Although many studies on FN in patients with breast cancer have been conducted with the AC-DTX regimen and have described the risk factors for FN with this regimen based on the statistical data, few have mentioned the underlying mechanisms of developing FN [12, 13]. Moreover, these studies focused on patients with FN, not patients with grade 3 neutropenia. Considering that coronavirus disease 2019 is prevalent all over the world [14] and that preventing severe neutropenia has become more important than ever, we focused on not only FN but also grade 3 neutropenia, which could delay treatment and lead to FN.
The current study aimed to reveal the characteristics of patients who need pegfilgrastim for primary prophylaxis of severe neutropenia, including FN and grade 3 neutropenia, during neoadjuvant AC-DTX chemotherapy. We demonstrated that higher stage of breast cancer is significantly associated with developing severe neutropenia in patients treated with neoadjuvant AC-DTX chemotherapy.
| Materials and Methods | ▴Top |
Ethics approval
This study was approved by the Institutional Review Board of the Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan (2018-0137). All patients included in this study provided written consent for treatment. Patients were not needed to provide well informed consent to this research because the evaluation applied unidentified clinical information. This study was conducted in compliance with ethical standards of the responsible institution on human subjects as well as with Helsinki Declaration.
Study cohort
One hundred thirty-eight patients with breast cancer received NAC and surgery at the Niigata University Medical and Dental Hospital between 2010 and 2019 (Fig. 1) were included in this study.
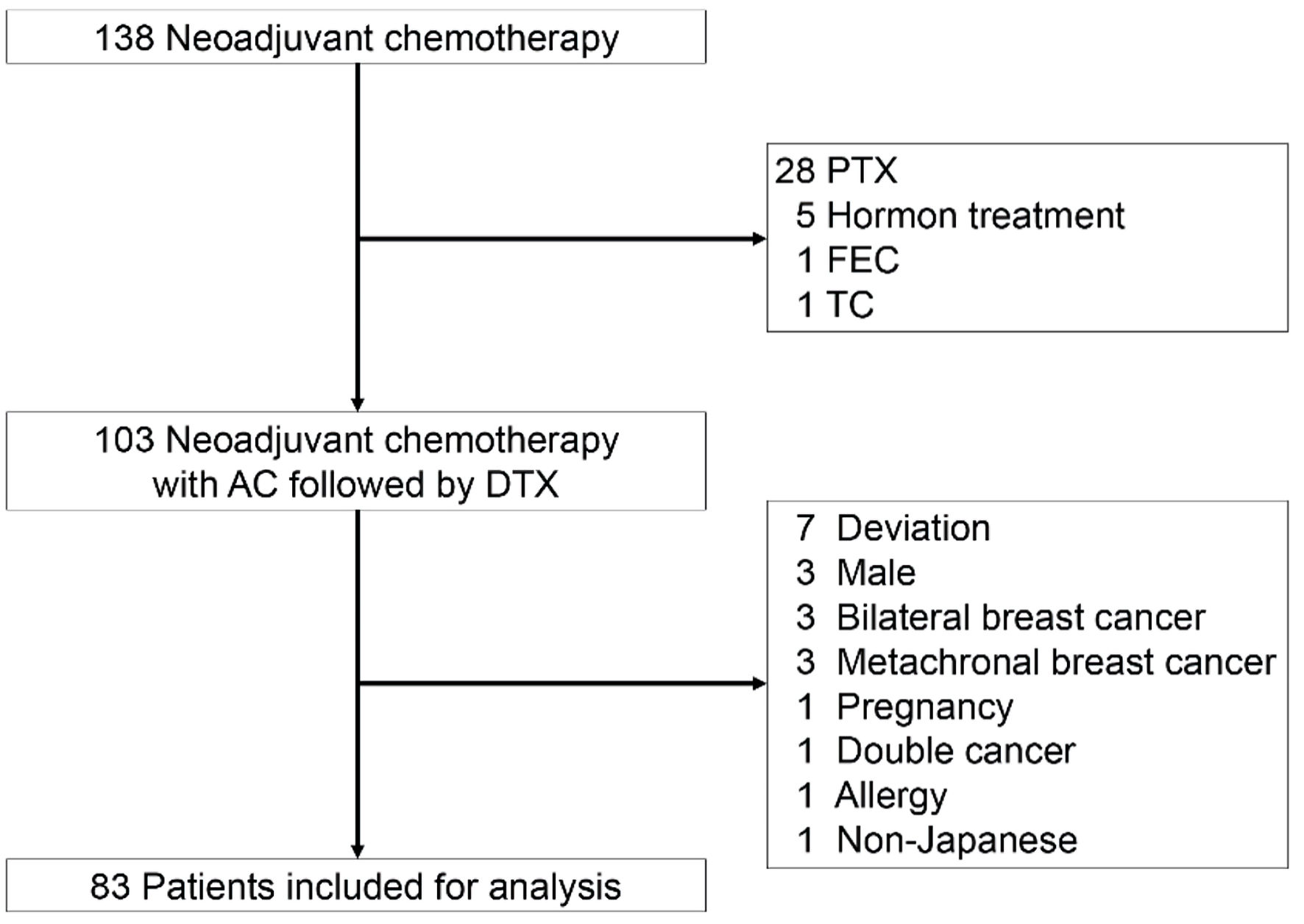 Click for large image | Figure 1. The CONSORT diagram with inclusion and exclusion criteria. Eighty-three patients were included for analysis. AC: adriamycin/cyclophosphamide; DTX: docetaxel; FEC: fluorouracil/epirubicin hydrochloride/cyclophosphamide; PTX: paclitaxel; TC: docetaxel/cyclophosphamide. |
Inclusion and exclusion criteria
Patients treated with NAC with AC-paclitaxel (PTX), fluorouracil/epirubicin hydrochloride/cyclophosphamide (FEC)-DTX, DTX/cyclophosphamide (TC), and in combination with hormone therapy were excluded (Fig. 1). Among 103 patients treated with neoadjuvant AC-DTX chemotherapy, patients with treatment deviations, bilateral breast cancer, metachronal breast cancer, pregnancy, double cancer, and allergies were excluded to minimize other confounding factors. Male patients and non-Japanese patients were also excluded.
Systemic treatment
The patients received four cycles of adriamycin (60 mg/m2) and cyclophosphamide (600 mg/m2) followed by four cycles of DTX (75 mg/m2) every 21 days. Blood counts were measured before each cycle by chemistry assays. The patients who were referred to our institution with fever or general malaise and diagnosed with FN or severe neutropenia (grade 3 and grade 4) were counted. According to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, we defined FN as neutropenia (< 500 neutrophils/mm3 or < 1,000 neutrophils/mm3 for over 48 h) with a febrile event (oral temperature ≥ 38.3 °C or ≥ 38.0 °C for over 1 h). Grade 3 neutropenia was defined as neutropenia (< 1,000 neutrophils/mm3). Administration of pegfilgrastim for prophylaxis and dose reductions/delays of the NAC were decided by the physicians respectively.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 23. We compared continuous variables with the Mann-Whitney U-test and categorical variables using Fisher’s exact test as the univariate analyses. A logistic regression model was used to identify the independent factors which were associated with severe neutropenia in the multivariate analysis. The variables with P values less than 0.10 in the univariate analyses were entered into the model. Two-sided P values less than 0.05 were considered statistically significant.
| Results | ▴Top |
The incidence of severe neutropenia in Japanese patients with breast cancer
A total of 83 Japanese patients with breast cancer receiving AC-DTX as NAC were included in the current study. Eighteen of 83 patients (22%) were diagnosed with severe neutropenia, including FN (Table 1). Twenty-two of 83 patients (27%) received pegfilgrastim for primary prophylaxis, and 61 of 83 patients (73%) did not receive pegfilgrastim for primary prophylaxis (Table 1). Severe neutropenia developed in one of 22 patients (5%) with pegfilgrastim for primary prophylaxis and in 17 of 61 patients (28%) without pegfilgrastim for primary prophylaxis. Severe neutropenia also developed during the AC regimen in 10 of 83 patients (12%) and during the DTX regimen in eight of 83 patients (10%).
 Click to view | Table 1. Univariate and Multivariate Analyses of the Factors Which Associated With Severe Neutropenia Among 83 Patients |
Pegfilgrastim for primary prophylaxis has a preventive effect on severe neutropenia
We confirmed the preventive effect of pegfilgrastim for primary prophylaxis on severe neutropenia. The univariate analysis showed that the incidence of severe neutropenia was significantly decreased in the patients with pegfilgrastim for primary prophylaxis (P = 0.023, Table 1). The multivariate analysis showed that pegfilgrastim for primary prophylaxis was associated with preventing from severe neutropenia in patients with breast cancer (odds ratio, 0.063; 95% confidence interval, 0.005 - 0.770, P = 0.030). In addition, tumor size was associated with developing severe neutropenia (odds ratio, 0.081; 95% confidence interval, 1.090 - 127.885, P = 0.042).
Advanced stage was significantly associated with development of severe neutropenia
Next, we explored the characteristics of patients which were associated with severe neutropenia in 61 patients without pegfilgrastim for primary prophylaxis to select the appropriate candidate for the primary prophylaxis. The univariate analysis showed that severe neutropenia was associated with tumor size (P = 0.004), clinical stage (P = 0.009), and cancer antigen 15-3 (CA15-3) (P = 0.026) (Table 2). The univariate analysis also showed that severe neutropenia tended to be associated with lymph node metastasis (P = 0.067) and carcinoembryonic antigen (CEA) (P = 0.088) (Table 2). The multivariate analysis showed that clinical stage was associated with developing severe neutropenia (odds ratio, 5.194; 95% confidence interval, 1.288 - 20.946, P = 0.021). Among 17 patients with severe neutropenia, severe neutropenia developed during the AC regimen in 10 of 17 patients (59%), whereas severe neutropenia developed during the DTX regimen in seven of 17 patients (41%). Clinical stage III tended to be associated with severe neutropenia in the earlier phase of chemotherapy (during the AC regimen), whereas clinical stage II tended to be associated with severe neutropenia in the later phase (during the DT regimen) (P = 0.059) (Table 3).
 Click to view | Table 2. Univariate and Multivariate Analyses for Severe Neutropenia Among 61 Patients Without Pegfilgrastim for Primary Prophylaxis |
 Click to view | Table 3. Association Between Clinical Stage and Period of Observed Severe Neutropenia in 17 Patients Without Pegfilgrastim for Primary Prophylaxis |
| Discussion | ▴Top |
NAC needs to be performed without adverse events including severe neutropenia to avoid delay of surgical treatment. Many guidelines, including those from the American Society of Clinical Oncology [6], National Comprehensive Cancer Network [7], and European Platform of Cancer Research [8], have classified AC regimens into the intermediate-risk group for FN, and patients are selected for primary prophylaxis with pegfilgrastim based on risk indices such as that from the Multinational Association for Supportive Care in Cancer (MASCC) [15]. However, we sometimes found patients with severe neutropenia whose conditions did not meet these standards. The current study showed that higher stage of breast cancer is significantly associated with developing severe neutropenia during NAC among patients without pegfilgrastim for primary prophylaxis. Our results indicated the importance of recognizing tumor factors when selecting patients for primary prophylaxis with pegfilgrastim during neoadjuvant AC-DTX chemotherapy.
The current study demonstrated that higher clinical stage was associated with developing severe neutropenia in patients with breast cancer during neoadjuvant AC-DTX chemotherapy. A previous study reported that patients with metastatic breast cancer developed FN more easily than patients with early breast cancer during AC-DTX chemotherapy [16]. It has also been reported that there are more FN events in patients treated with NAC than in those treated with adjuvant chemotherapy and that NAC was an independent risk factor for FN [17]. These results indicate that patients with a higher tumor burden may be associated with the development of FN during chemotherapy. The current study also demonstrated that patients with a higher tumor burden (clinical stage III) tended to develop severe neutropenia earlier than patients with less tumor burden (clinical stage II) among the group of patients with severe neutropenia (Table 3). Taken together, the higher tumor burden might contribute to the development of neutropenia during NAC.
How does tumor burden affect the host to promote neutropenia development during NAC? Tumor microenvironment (TME), composed with different cell types such as neutrophils, macrophages, and monocytes, produces a variety of chemical mediators including cytokines, growth factor, exosomes and chemokines, and these mediators affect on cell differentiation and/or self-renewal of hematopoietic stem cells [18-20]. For example, myeloid-derived suppressor cell (MDSC), which is one of the major tumor-infiltrating myeloid cells, influences the hematopoietic stem cells [21]. The mouse 4T1 breast cancer cells released colony-stimulating factor-1 (CSF-1), leading to promote bone marrows output of MDSCs [22]. Chemotherapy produced CSF-2 from various cancers, leading to induce the differentiation of monocytes into MDSCs in bone marrow [23]. TME could threaten the homeostasis of the stem cell niche during NAC (Fig. 2). Based on this hypothesis, it is also reasonable that cancer stage is not associated with neutropenia in patients treated with adjuvant chemotherapy (Fig. 2) [24, 25].
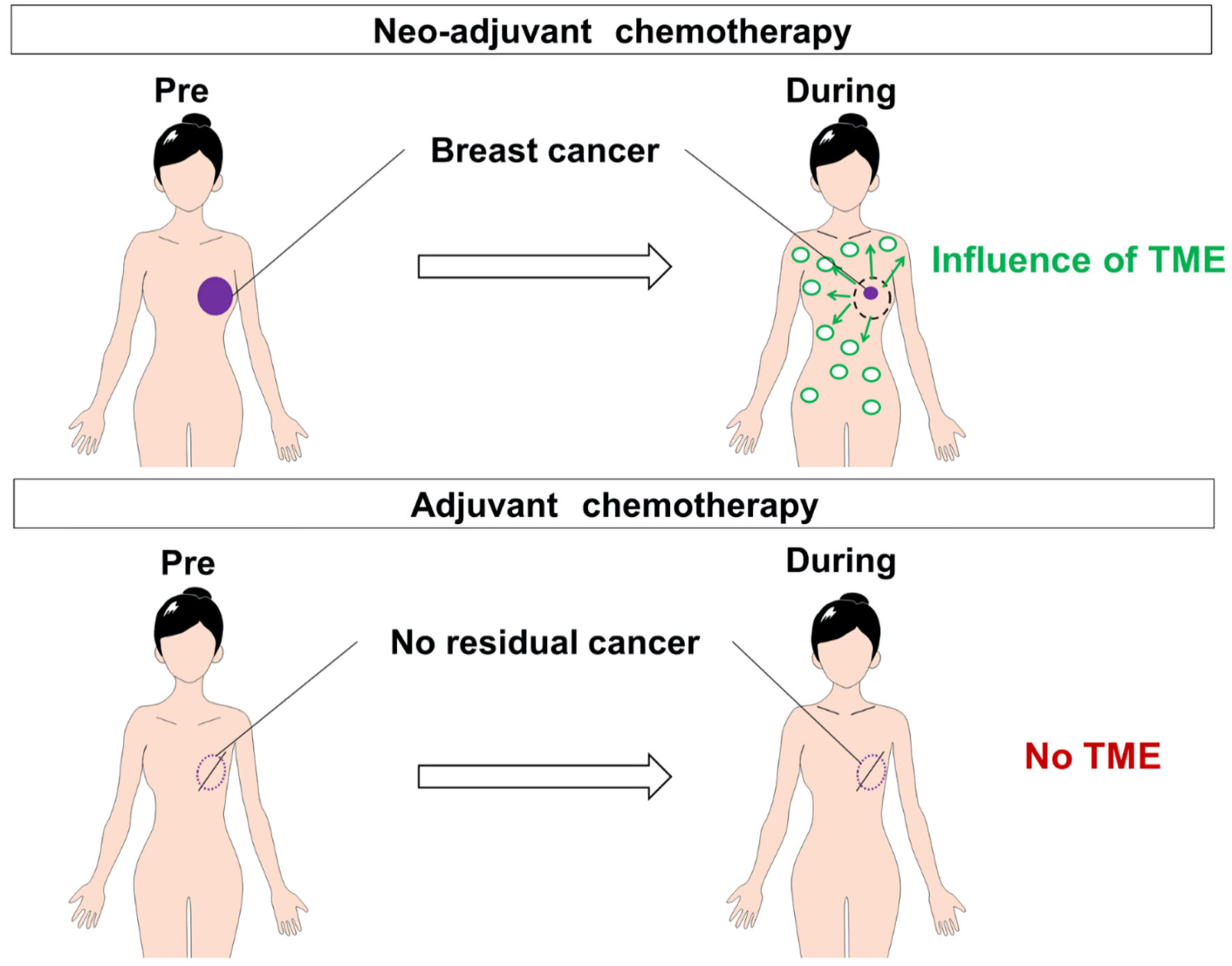 Click for large image | Figure 2. The comparison of responses after chemotherapy between patients treated with neoadjuvant chemotherapy and those treated with adjuvant chemotherapy. There were plenty of TME-derived soluble and bioactive factors (cytokines, growth factors, exosomes, and chemokines) during neoadjuvant chemotherapy, whereas there were no TME in adjuvant chemotherapy. TME: tumor microenvironment. |
There are limitations in the current study. First, this was a single-institution retrospective analysis with a small number of patients; however, we think the selection bias is minimal because consecutive breast cancer patients treated with neoadjuvant AC-DTX chemotherapy at our institution between 2010 and 2019 were enrolled. Second, we registered patients with severe neutropenia by patient consultation. It is possible that we underestimated the number of patients with mild neutropenia, since we did not include patients who did not visit our hospital due to their mild symptoms. Importantly, however, multivariate analysis revealed that clinical stage was significantly associated with developing severe neutropenia in our study. In conclusion, patients with higher clinical stage of breast cancer might have to be considered for primary prophylaxis with pegfilgrastim during neoadjuvant AC-DTX chemotherapy.
Acknowledgments
We appreciate the support of the Niigata University Hospital.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
M. Nagahashi received honoraria from Kyowa Kirin Co., Ltd.
Informed Consent
Written informed consent of all cases was obtained for both study and publication.
Author Contributions
K. Moro, M. Nagahashi, Y. Koyama and K. Takabe contributed to the conception and design of the study. K. Moro, M. Nagahashi, and H. Ichikawa contributed to analysis and interpretation of data. K. Moro, H. Uchida, M. Oji, J. Tsuchida, K. Yamaura, C. Toshikawa, M. Nakano and M. Ikarashi contributed to collection and assembly of data. K. Moro, M. Nagahashi, Y. Muneoka, Y. Tajima, Y. Shimada, J. Sakata, K.Takabe and T. Wakai contributed to drafting of the article. T. Wakai gave final approval for submission of the article.
Data AvailabilityThe authors declare that data supporting the findings of this study are available within the article.Abbreviations
AC: adriamycin/cyclophosphamide; CR: complete response; CSF: colony-stimulating factor; CTCAE: Common Terminology Criteria for Adverse Events; DTX: docetaxel; FEC: fluorouracil/epirubicin hydrochloride/cyclophosphamide; FN: febrile neutropenia; NAC: neoadjuvant chemotherapy; PR: partial response; PTX: paclitaxel; RECIST: Response Evaluation Criteria in Solid Tumors; SD: stable disease; TAC: docetaxel, adriamycin and cyclophosphamide; TC: docetaxel/cyclophosphamide; TME: tumor microenvironment
| References | ▴Top |
- Asaoka M, Gandhi S, Ishikawa T, Takabe K. Neoadjuvant chemotherapy for breast cancer: past, present, and future. Breast Cancer (Auckl). 2020;14:1178223420980377.
doi pubmed - Olfa G, Amel T, Rim C, Aymen Z, Faten E, Makrem H, Leila BF, et al. Clinical and pathological response to neoadjuvant anthracycline based chemotherapy in women with breast cancer. World J Oncol. 2010;1(4):167-172.
- Djulbegovic B, Norris LB, Bennett CL. Colony-stimulating factors for febrile neutropenia. N Engl J Med. 2013;369(3):286.
doi - Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266.
doi pubmed - Miyake O, Murata K, Tanaka S, Ishiguro H, Toi M, Tamura K, Kawakami K. Costs associated with febrile neutropenia in Japanese patients with primary breast cancer: post-hoc analysis of a randomized clinical trial. Jpn J Clin Oncol. 2018;48(5):410-416.
doi pubmed - Smith TJ, Bohlke K, Armitage JO. Recommendations for the use of white blood cell growth factors: american society of clinical oncology clinical practice guideline update. J Oncol Pract. 2015;11(6):511-513.
doi pubmed - Becker PS, Griffiths EA, Alwan LM, Bachiashvili K, Brown A, Cool R, Curtin P, et al. NCCN Guidelines Insights: Hematopoietic Growth Factors, Version 1.2020. J Natl Compr Canc Netw. 2020;18(1):12-22.
doi pubmed - Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8-32.
doi pubmed - Jenkins P, Scaife J, Freeman S. Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer. Ann Oncol. 2012;23(7):1766-1771.
doi pubmed - Hurria A, Brogan K, Panageas KS, Jakubowski A, Zauderer M, Pearce C, Norton L, et al. Change in cycle 1 to cycle 2 haematological counts predicts toxicity in older patients with breast cancer receiving adjuvant chemotherapy. Drugs Aging. 2005;22(8):709-715.
doi pubmed - Vaid AK, Khurana A, Sharma D, Gautam D, Wadhwa J, Agarwal R, Kaur K, et al. Clinical characteristics and outcome trends of adjuvant anthracycline and Taxane regimen for early stage breast cancer. World J Oncol. 2020;11(3):106-111.
doi pubmed - Kim CG, Sohn J, Chon H, Kim JH, Heo SJ, Cho H, Kim IJ, et al. Incidence of febrile neutropenia in Korean female breast cancer patients receiving preoperative or postoperative doxorubicin/cyclophosphamide followed by docetaxel chemotherapy. J Breast Cancer. 2016;19(1):76-82.
doi pubmed - Hosmer W, Malin J, Wong M. Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer. 2011;19(3):333-341.
doi pubmed - Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
doi - Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, Gallagher J, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18(16):3038-3051.
doi pubmed - Perez EA, Geeraerts L, Suman VJ, Adjei AA, Baron AT, Hatfield AK, Maihle N, et al. A randomized phase II study of sequential docetaxel and doxorubicin/cyclophosphamide in patients with metastatic breast cancer. Ann Oncol. 2002;13(8):1225-1235.
doi pubmed - Baghlaf SS, Abulaban AA, Abrar MB, Al-Shehri AS. Chemotherapy-induced febrile neutropenia in patients with breast cancer. A multivariate risk assessment model for first cycle chemotherapy. Saudi Med J. 2014;35(6):612-616.
- Tanaka T, Bai Z, Srinoulprasert Y, Yang BG, Hayasaka H, Miyasaka M. Chemokines in tumor progression and metastasis. Cancer Sci. 2005;96(6):317-322.
doi pubmed - Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436-444.
doi pubmed - Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, Viola S, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146(5):598-604.
doi pubmed - De Sanctis F, Adamo A, Cane S, Ugel S. Targeting tumour-reprogrammed myeloid cells: the new battleground in cancer immunotherapy. Semin Immunopathol. 2022;26:1-24.
doi pubmed - Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248-21255.
doi pubmed - Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, et al. Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res. 2015;75(13):2629-2640.
doi pubmed - Chan A, Chen C, Chiang J, Tan SH, Ng R. Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer. 2012;20(7):1525-1532.
doi pubmed - Kim HS, Lee SY, Kim JW, Choi YJ, Park IH, Lee KS, Seo JH, et al. Incidence and predictors of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy in Korea. Oncology. 2016;91(5):274-282.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


