| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 6, December 2022, pages 343-349
The Association Between Aspirin and Basal Cell Carcinoma: A Clinical and Financial Analysis
Lexi Frankela, Amalia D. Ardeljanb, Kazuaki Takabec, d, Omar M. Rashida, b, e, f, g, h, i, j, k
aNova Southeastern University, Dr. Kiran C. Patel College of Allopathic Medicine, Fort Lauderdale, FL, USA
bMichael and Dianne Biennes Comprehensive Cancer Center, Holy Cross Health, Fort Lauderdale, FL, USA
cDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
dDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY, USA
eLeonard Miami School of Medicine, University of Miami, Miami, FL, USA
fMassachusetts General Hospital, Boston, MA, USA
gBroward Health, Fort Lauderdale, FL, USA
hTopLine MD Alliance, Fort Lauderdale, FL, USA
iMemorial Health, Pembroke Pines, FL, USA
jDelray Medical Center, Delray, FL, USA
kCorresponding Author: Omar M. Rashid, Complex General Surgical Oncology, General & Robotic Surgery, TopLine MD Alliance, Fort Lauderdale, FL 33308, USA
Manuscript submitted September 30, 2022, accepted November 5, 2022, published online December 1, 2022
Short title: Aspirin and Basal Cell Carcinoma Link
doi: https://doi.org/10.14740/wjon1533
| Abstract | ▴Top |
Background: Nonmelanoma skin cancer (NMSC) is the most common malignancy. Basal cell carcinoma (BCC) comprises about 80% of all NMSCs and its incidence continues to rise. Although BCC rarely leads to metastases or increased mortality, its effects on healthcare costs and quality of life are substantial. Aspirin may prevent the development of basal cell carcinoma (BCC) by the inhibition of cyclooxygenase (COX) enzymes, which are associated with carcinogenesis and inflammation. This study therefore examined the effect of aspirin on the risk of BCC, its clinical outcomes, and its treatment costs.
Methods: A retrospective study (2010 - 2018) was conducted using the Humana Health Insurance Database. International Classification of Disease ninth and 10th codes and National Drug Codes were used to identify BCC diagnoses and aspirin prescriptions. Patients were matched for age, sex, Charlson Comorbidity Score (CCI), and region of residence. Chi-squared, logistic regression, and odds ratio (OR) analyses were utilized to test for significance and to estimate relative risk.
Results: Aspirin use was associated with a decreased incidence of BCC in unmatched (OR = 0.658, 95% confidence interval (CI) 0.526 - 0.820) and matched (OR = 0.54, 95% CI 0.47 - 0.61) analyses. Aspirin was also associated with a decreased BCC risk when stratified by hypertension (P = 3.888 × 10-5), chronic obstructive pulmonary disease (COPD) (P = 0.014), diabetes (P = 0.049) and tobacco use (P = 0.017). Aspirin use was not associated with risk of BCC when stratified by obesity (P = 0.408). The average paid per patient for BCC treatment was significantly higher for patients in the aspirin use group than in the aspirin nonuse group (P = 0.0087).
Conclusions: While the high incidence and cost of treatment of BCC are demanding both clinically and financially, the low cost of aspirin and its widespread use may have vital implications for its preventative role in this disease. This study concluded that aspirin use was associated with a significantly decreased risk of BCC.
Keywords: Aspirin; Basal cell carcinoma; Nonmelanoma skin cancer; Ultraviolet exposure; COX-1; COX-2
| Introduction | ▴Top |
Nonmelanoma skin cancer (NMSC) is the most common malignancy [1]. Basal cell carcinoma (BCC) comprises about 80% of all NMSCs and it is rising [2]. The highest rates are currently seen in elderly men and the incidence of BCC is increasing in the younger population, especially women [3]. BCC pathogenesis is dependent on various environmental and genetic factors, including ultraviolet (UV) exposure, diet, and aberrant signaling pathways [4]. Although BCC rarely leads to metastases or increased mortality, its effects on healthcare costs and quality of life are substantial [5]. In particular, recurrence of BCC may cause functional and aesthetic complications, which further impinge upon quality of life [6]. Further, treatment of BCC may include expensive and labor-intensive modalities such as Mohs micrographic surgery surgical excision, radiotherapy, or topical medications [7]. These treatments alone are not only extremely costly but may also require consistent follow-up that further perpetuates the financial burden of this disease [6].
Although treatment of BCC is costly, only few chemopreventive therapies have been identified and further preventative approaches are crucial [8]. Recent literature has identified the potential role of non-steroidal anti-inflammatory drugs (NSAIDs) in decreasing the risks of various malignancies including BCC [9, 10]. This is due to their role of decreasing the inflammation involved in the propagation of BCC and other epithelial cell tumors [11]. Aspirin inhibits both cyclooxygenase (COX)-1 and COX-2, with stronger inhibitory effects on COX-1 [12]. Additionally, the administration of aspirin is associated with the prevention of atherothrombosis through the inhibition of platelet COX-1 activity, which is associated with neoplastic transformation [13]. COX-2, which is upregulated by type B ultraviolet (UVB) exposure, increases prostaglandin synthesis and subsequent inflammation [11]. As UVB exposure is a major risk factor for the development of BCC, upregulation of COX-2 may be implicated in its development and its inhibition a possible preventative route [14]. Previous studies have also identified the tumorigenic role of COX-2 in the downregulation of apoptosis and upregulation of angiogenesis in skin cancer [15]. Although the chemopreventive effects of aspirin for development of BCC have been previously studied, the results remain controversial in the literature [10, 16-24]. While several studies report a negative association between aspirin use and development of BCC, others report a positive association, and yet others are inconclusive [10, 11, 13-20].
Prior studies do not provide a complete analysis of the prognostic variables and cost-effectiveness associated with the use of aspirin for the treatment or prevention of BCC (Table 1) [10, 16-24]. As the majority of the literature is self-reported, there is a significant lack of data that has been previously associated with outcome reporting bias [25, 26]. Due to the heterogeneity of literature outcomes and incomplete reporting, variables such as site of origin and aspirin dosage have been missing or unable to be analyzed adequately in meta-analyses. Additionally, several studies only include patients from a single center or state or have been conducted internationally, decreasing their external validity to a wide range of patients. This retrospective study was performed to analyze the prognostic variables of aspirin use and its effects on the clinical and financial outcomes of BCC. This is the first study to directly analyze the effects of aspirin use on the cost of treatment and to analyze aspirin alone (without an adjunctive analysis of other medications) on its effect on BCC development.
 Click to view | Table 1. A Literature Review on Aspirin and Basal Cell Carcinoma Detailing the Inclusion of Various Criteria in Previous Studies |
| Materials and Methods | ▴Top |
This study was conducted using a Health Insurance Portability and Accountability act compliant National Database. The Patient Population Database in the USA was retrospectively reviewed from 2010 to 2018 using the International Classification of Disease (ICD) ninth and 10th Revision diagnostic codes for BCC and generic drug codes for aspirin. The index date was selected using the available data in the national database. We used the earliest possible date that included enough patient information to make relevant analyses. This analysis of the data was conducted in a two-step process to assess the effect of demographic data and confounding variables on outcome measures. First, the Humana database was analyzed using comparison groups unmatched for patient characteristics. In the second step, the Humana database was analyzed again using comparison groups matched for age, sex, Charlson Comorbidity Score (CCI), and region of residence.
The results from the first and second outputs were then compared to assess the effects of demographic data on statistical significance and outcome measures. The unmatched query yielded 16,198 patients taking aspirin and 1,545,183 not taking aspirin. The matched query yielded 16,198 patients taking aspirin and 16,198 patients not taking aspirin (Fig. 1).
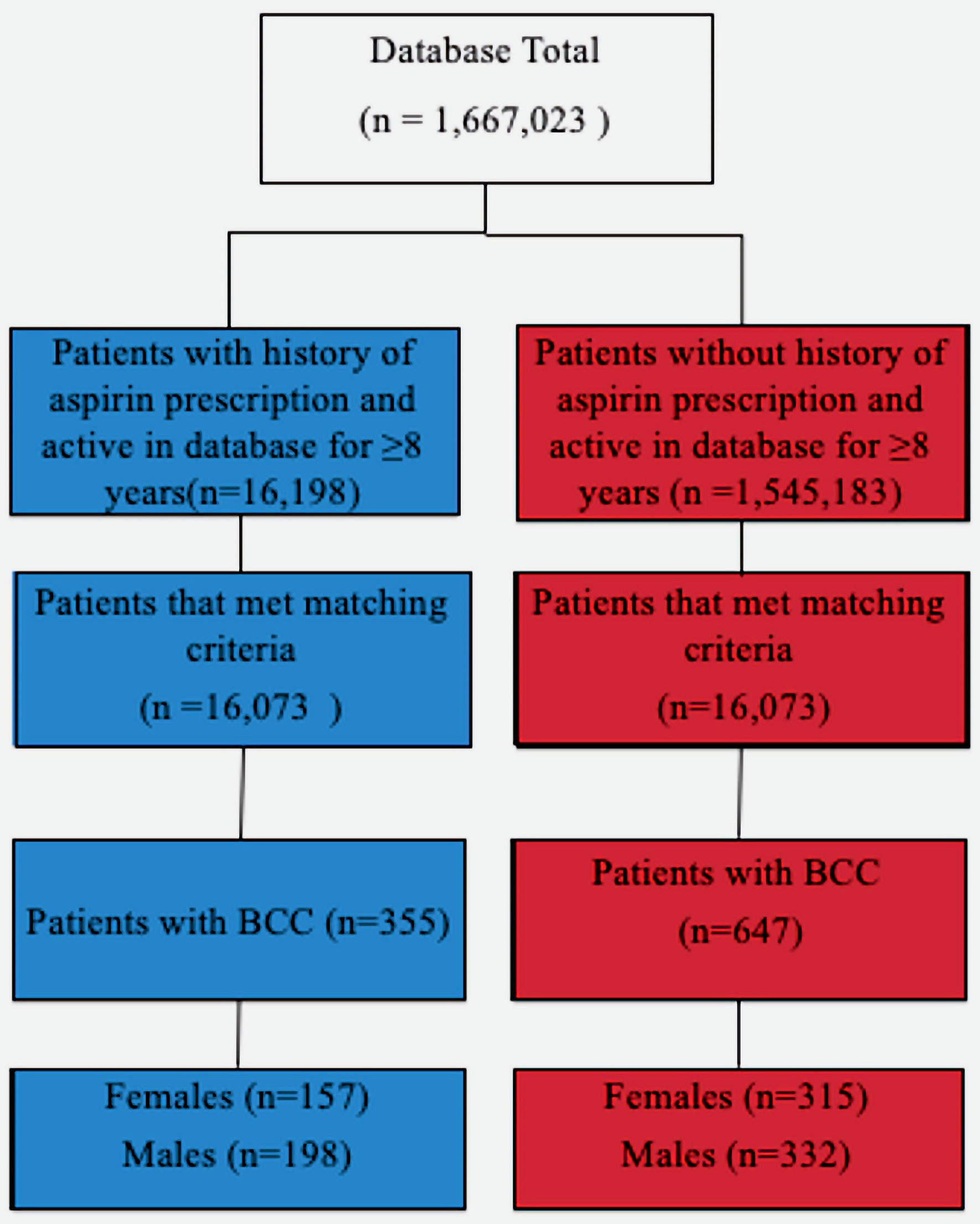 Click for large image | Figure 1. Diagram depicting the grouping of patients based on various criteria. BCC: basal cell carcinoma. |
Users of aspirin were defined as patients with an active aspirin prescription for a minimum of 8 years. Incident or one-time prescription users of aspirin were not included. Patients who stopped taking aspirin were excluded from the study. Inclusion in the aspirin group required actively taking aspirin for a minimum of 8 years. There were several indications for which patients were taking aspirin, although the majority were cardiac and pain. The data were then stratified by comorbidities to assess for differences for patients with hypertension, obesity, diabetes, chronic obstructive pulmonary disease (COPD), and tobacco use. Healthcare costs were compared both between aspirin use and nonuse groups, and between patients with and without hemorrhagic complications in the aspirin use group. Analysis of healthcare costs for aspirin vs. no aspirin aimed at understanding differences in BCC severity and required treatment. The costs are specific for patients being treated specifically for BCC. Healthcare data were analyzed using terminology provided by the Pearl Diver User Manual, the statistical program used for our analysis (Table 2). Chi-squared analyses were used to test for significant relationships between populations using and not using aspirin containing drugs. Logistic regression and odds ratio (OR) calculations were utilized to assess the risk of developing BCC with aspirin use. To avoid attrition bias, patients that were from the aspirin use and nonuse populations that were lost to follow-up were automatically excluded from our study.
 Click to view | Table 2. Descriptions of Financial Terms as Provided by PearlDiver National Database User Manual |
This study was exempted from the institutional review board (IRB) approval because of the database study and was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patient demographics
For the first step of the unmatched analysis, the Humana database yielded 16,198 patients with an active aspirin prescription. Of those patients, 360 (2.22%) also had a BCC diagnosis. A total of 1,545,183 patients were identified as not having an active aspirin prescription and of those patients, 50,547 (3.27%) had a BCC diagnosis. Overall, men accounted for a greater proportion of BCC in both patients taking (56%) and not taking aspirin (56%). In both aspirin use and nonuse groups, the largest percentage of patients was between the ages of 70 - 74, followed by 75 - 79 and 65 - 69. The Southern United States demonstrated the highest percentage of patients in both aspirin use and nonuse groups and the Western United States had the lowest percentage of patients.
In the second step, patients were matched for age, sex, CCI, and region of residence, and were divided into two equal groups (aspirin use and aspirin nonuse) of 16,073 patients each. Males accounted for a larger percentage of patients with BCC in both aspirin use (55.77%) and aspirin nonuse (51.31%) groups. There was not a significant difference (P = 0.2007) between the ratio of males to females with BCC between the aspirin use population (198:157) and the aspirin nonuse population (332:315) (P = 0.198). For both aspirin use and nonuse populations with BCC, the skin of the face was the most commonly affected site, followed by the trunk (excluding the scrotum). There was not a significant difference between aspirin use and nonuse populations for CCI score (P = 0.302) and the 10-year percent survival rate was found to decrease as CCI score increased.
Aspirin and BCC
In the unmatched analysis, the use of aspirin was associated with a decreased risk of development of BCC compared to nonuse of aspirin (OR = 1.52, 95% CI 1.22 - 1.90). In the unmatched dataset, the percentage of patients with BCC was significantly lower (P = 9.03 × 10-14) by 1.07% in the patients taking aspirin compared to patients not taking aspirin (360/16,198 (2.22%) versus 50,547/1,545, 183 (3.27%)).
In the matched analysis, the use of aspirin was associated with an even further decreased risk of BCC (Fig. 2). Aspirin use was associated with a decreased incidence of BCC in unmatched (OR = 0.658, 95% CI 0.526 - 0.820) and matched (OR = 0.54, 95% CI 0.47 - 0.61) analyses. Aspirin was also associated with a decreased BCC risk when stratified by hypertension (P = 3.888 × 10-5), COPD (P = 0.014), diabetes (P = 0.049) and tobacco use (P = 0.017). Aspirin use was not associated with risk of BCC when stratified by obesity (P = 0.408). The average paid per patient for BCC treatment was significantly higher for patients in the aspirin use group than in the aspirin nonuse group (P = 0.0087). Of the 360 patients from the aspirin use group with BCC, 20 patients (5.56%) experienced a bleeding complication.
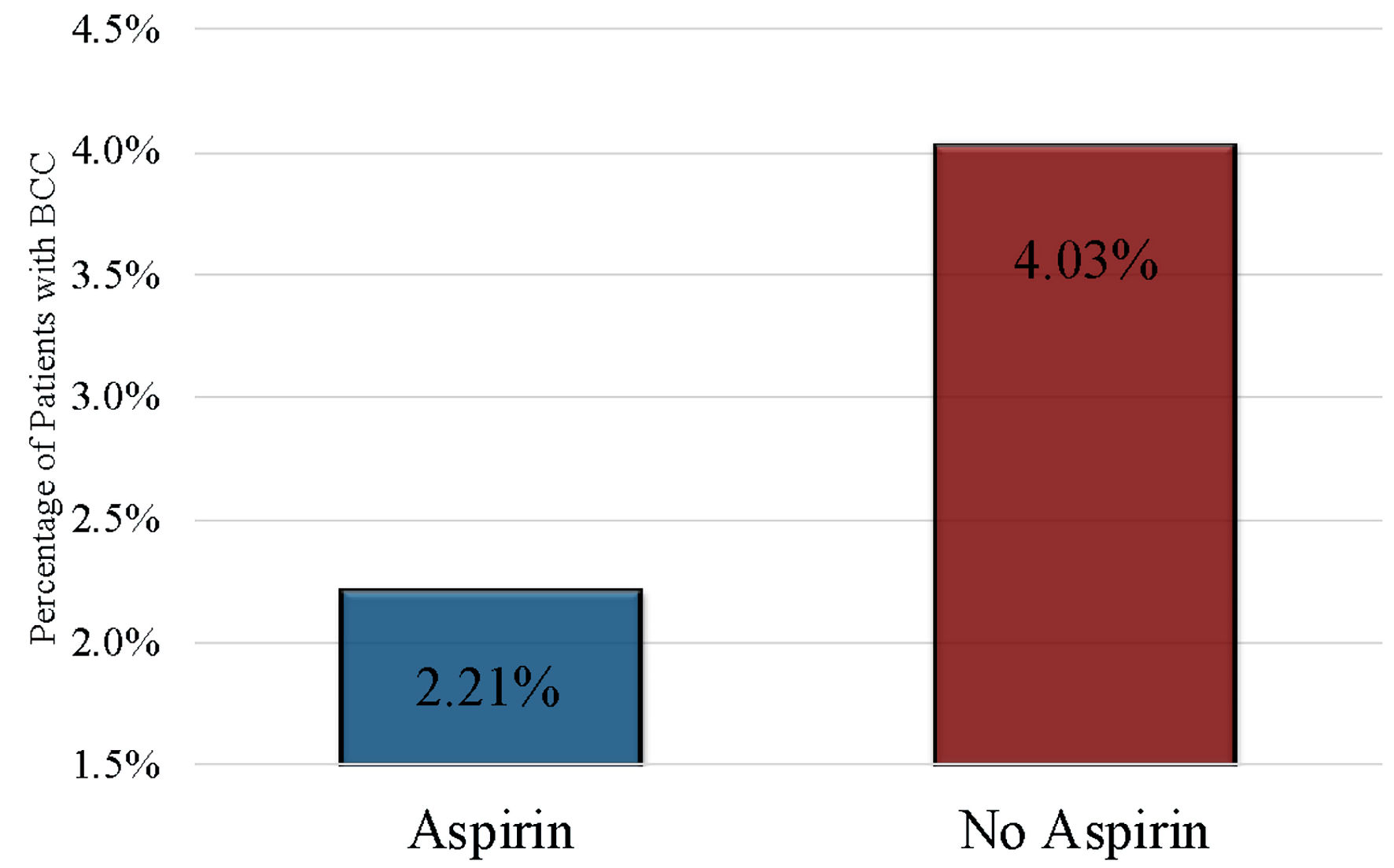 Click for large image | Figure 2. Percentage of patients with BCC compared by aspirin use and nonuse (P < 2.2 × 10-16, OR = 0.54, 95% CI 0.47 - 0.61). BCC: basal cell carcinoma; OR: odds ratio; CI: confidence interval. |
Healthcare costs
The analysis of financial data and length of stay (LOS) was conducted in the second step of this study utilizing matched data. For patients with BCC, average paid per claim, event, and patient were higher in the aspirin use group than the aspirin nonuse group (Fig. 3). For the 5.56% of patients that experienced bleeding complications in the aspirin use group, total healthcare costs were higher than average total healthcare costs. Healthcare costs for BCC treatment varied between patients with comorbidities (COPD, diabetes, hypertension, obesity, and tobacco use) and the total population of patients with BCC. In the aspirin use group, the average paid per event ($ 128.93) and average paid per claim ($133.93) were higher in patients with hypertension than both the other comorbidities and the total ($75.30 and $75.30). Average paid per patient was highest in patients with diabetes ($391.35) and was $317.09 higher than the total average ($74.26). In the aspirin nonuse group, average paid per claim ($177.43), event ($187.87), and patient ($270.06) were all higher in patients with diabetes than other comorbidities, and than the total averages ($67.23, $67.23, and $43.69, respectively).
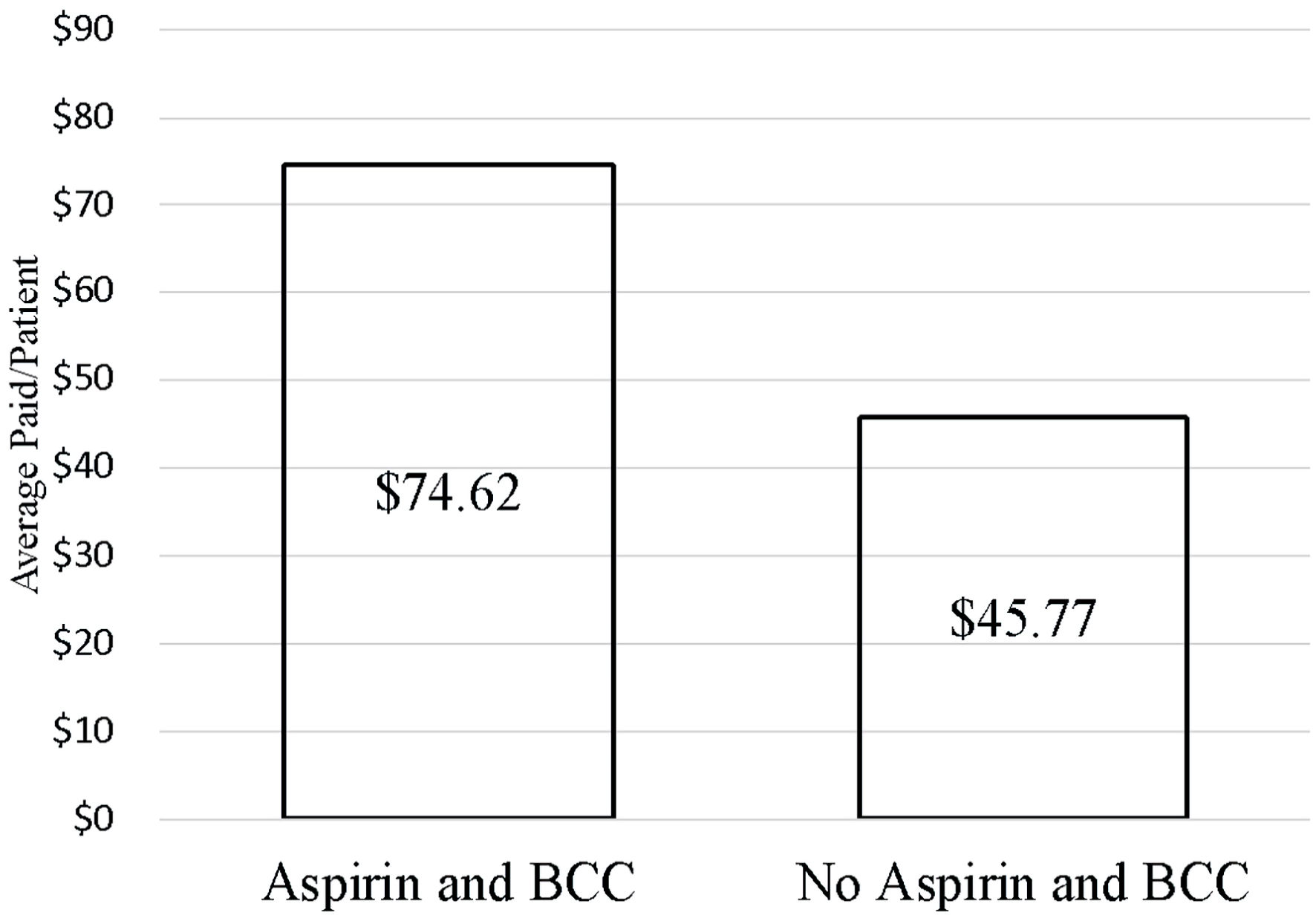 Click for large image | Figure 3. Average paid per patient for BCC treatment compared between aspirin use and nonuse (P = 0.0087). BCC: basal cell carcinoma. |
For patients with BCC in the aspirin use group, average LOS was highest for patients with diabetes (6.02 days) and lowest for patients with COPD (3.64 days). For patients with BCC in the aspirin nonuse group, average LOS was highest for patients with obesity (7.88 days) and lowest for patients with COPD (4.94 days).
| Discussion | ▴Top |
This retrospective study demonstrates the role of aspirin in decreasing the risk of BCC. This result is consistent with other similar studies, although differs from several others which report no association or a positive association between aspirin and BCC (Table 1) [10, 16-24]. However, this study included an analysis of variables inadequately examined in the literature previously and was conducted with a large sample size, a diverse population, and propensity matching for confounding variables. Such measures were conducted to reduce the effects of reporting bias, confounding comorbidities and medications, and small and non-diverse populations. In this study, recall and reporting bias were avoided because all clinical data were extracted from automatically recorded prescriptions using National Drug Codes (NDC) in the database. In comparison, much of the existing literature on this field of study relies on self-reported questionnaires, which depend on patient recollection of diagnoses and medications from several years prior (Table 1) [10, 16-24].
Analysis of the data was conducted in two phases with matched and unmatched comparison groups to assess the effects of demographic data and confounding variables on outcome measures. In the first phase of the analysis, the data yielded significant results for the effect of aspirin on BCC development. In the second phase, when controls were matched for age and sex, aspirin use yielded an even stronger preventative role in BCC development. The risk of BCC development being more decreased as a result of aspirin use in the matched study versus the unmatched study shows that age and sex are likely confounders in the first phase of this study and potentially other similar studies as well. This finding might explain the positive or lack of association between aspirin and BCC previously noted in some studies, especially those conducted using unmatched control and experimental groups.
This study also analyzed the financial burden of BCC and its relationship to aspirin consumption, which has not been previously examined in the literature. The USA spends more on treatment of keratinocyte cancers than any other country [27], and the average annual cost of non-melanoma skin cancer treatment is estimated at $4.8 billion and increasing yearly [28]. In this study, the total cost of treatment for all patients with BCC was $53,691, and among those patients with BCC, the aspirin use group demonstrated higher healthcare costs than the aspirin nonuse group (Fig. 3). However, because 5.56% of patients in the aspirin use group developed bleeding complications, the higher average healthcare cost could be a result of managing these complications, rather related to management of BCC. Previous studies have demonstrated that at small doses, bleeding risk of aspirin is minimal. In order to assess if a small dose of aspirin would decrease bleeding complications while preventing BCC or decreasing its severity, future studies analyzing the dosage effects of aspirin on BCC are suggested. As previously discussed, the regular use of aspirin has been associated with hemorrhagic adverse effects. Its use for primary prevention for colorectal cancer has been questioned because of this complication, and some studies have therefore examined the role of aspirin for secondary prevention [29]. Similarly, in addition to the role of aspirin for primary prevention of BCC, further studies examining its role in secondary prevention are suggested.
The healthcare costs associated with various comorbidities was also examined for patients with BCC. For patients with BCC in the aspirin use group, average paid per event and claim was highest for patients with hypertension, and average cost per patient was highest for patients with diabetes. Similarly, for patients with BCC in the aspirin nonuse group, all average costs were highest for patients with diabetes. From a financial perspective, patients with hypertension or diabetes might be of special interest for aspirin for the prevention of BCC to avoid the increased cost of BCC treatment seen with these comorbidities. In a previous study analyzing the use of aspirin 325 mg for colorectal cancer prevention, the cost of aspirin therapy plus aspirin-related complications was reported to be $172 per year per patient [30]. Given that in this study, the average paid per patient per visit was $60.48 for patients with BCC, it would only require about three visits per year before the use of aspirin for primary prevention was cost-effective. Considering that many patients with BCC require consistent follow-up and continued monitoring, it is common for patients to visit their providers multiples times per year. Additionally, the aforementioned colorectal cancer study analyzed 325 mg aspirin, although this study included patients on 81 mg aspirin, from which it could be expected a lower cost and complication rate.
Although both aspirin use and nonuse groups contained a higher percentage of females (61%), both groups demonstrated a higher percentage of males with BCC (55.83% and 51.38% respectively.) As this study consisted mainly of older patients, our results are consistent with recent epidemiological studies which report that in older populations, the incidence of BCC is higher in males than females, which could be the result of women, on average, paying more attention to their skin’s health and appearance and subsequently, scheduling more medical appointments [31].
Several limitations in this study warrant a proper discussion. The observational retrospective design made it difficult to adjust for all potential and unknown confounding variables. While the data were matched for age and sex between the aspirin use and nonuse groups, there are several other factors that could confound outcome measures. While the database provided precise pharmaceutical information about official aspirin prescriptions, it lacked information regarding over the counter (OTC) aspirin use. However, the long-term use of OTC aspirin for chronic disease is restricted because it is generally more expensive and not subject to insurance reimbursement. We also lacked information regarding dosage and prescription lengths and dose-response effects were therefore unable to be analyzed. We also did not have information regarding lifestyle factors, individual UV exposure, or family history of skin cancer. As chronic UV exposure is a significant risk factor for the development of BCC, this is a limitation of our study that should be considered. However, stratification of the data based on the region of residence in the USA served to minimize the effects of varying UV exposures because BCC risk is strongly inversely correlated to geographic latitude [32]. Additionally, aspirin was prescribed for different reasons for each patient. While the majority of prescriptions were cardiac in nature, our study is limited by the fact that the conditions for which aspirin was taken could have affected the risk of BCC development. For this concern, we matched by the CCI to reduce the risk of this bias.
Conclusions
While the high incidence and cost of treatment of BCC are demanding both clinically and financially, the low cost of aspirin and its widespread use may have vital implications for its preventative role in this disease. This study concluded that aspirin use significantly decreased the risk of BCC.
Acknowledgments
The authors acknowledge the support of Holy Cross Hospital and Nova Southeastern University Dr. Kiran C. Patel College of Allopathic Medicine.
Financial Disclosure
Grant support from Broward Community Foundation.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Not applicable because of the database study.
Author Contributions
Authors Lexi Frankel is responsible for data extracting, analysis, manuscript writing, figures, and literature review. Amalia D. Ardeljan is responsible for data extracting and analysis. Authors Kazuaki Takabe and Omar M. Rashid are responsible for manuscript writing, editing, and data analysis.
Data Availability
Data were extracted from PearlDiver national database. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Didona D, Paolino G, Bottoni U, Cantisani C. Non melanoma skin cancer pathogenesis overview. Biomedicines. 2018;6(1):6.
doi pubmed - Bertozzi N, Simonacci F, Greco MP, Grignaffini E, Raposio E. Single center evidence for the treatment of basal cell carcinoma of the head and neck. Acta Biomed. 2019;90(1):77-82.
- Dika E, Scarfi F, Ferracin M, Broseghini E, Marcelli E, Bortolani B, Campione E, et al. Basal cell carcinoma: a comprehensive review. Int J Mol Sci. 2020;21(15):5572.
doi pubmed - Fania L, Didona D, Morese R, Campana I, Coco V, Di Pietro FR, Ricci F, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. biomedicines. 2020;8(11):449.
doi pubmed - Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
doi pubmed - Lanoue J, Goldenberg G. Basal cell carcinoma: a comprehensive review of existing and emerging nonsurgical therapies. J Clin Aesthet Dermatol. 2016;9(5):26-36.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88(2):167-179.
- van der Pols JC, Heinen MM, Hughes MC, Ibiebele TI, Marks GC, Green AC. Serum antioxidants and skin cancer risk: an 8-year community-based follow-up study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1167-1173.
doi pubmed - Patrignani P, Patrono C. Aspirin and Cancer. J Am Coll Cardiol. 2016;68(9):967-976.
doi pubmed - Jeter JM, Han J, Martinez ME, Alberts DS, Qureshi AA, Feskanich D. Non-steroidal anti-inflammatory drugs, acetaminophen, and risk of skin cancer in the Nurses' Health Study. Cancer Causes Control. 2012;23(9):1451-1461.
doi pubmed - Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19(5):723-729.
doi pubmed - Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101(10):1206-1218.
doi pubmed - Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373-2383.
doi pubmed - Madan V, Hoban P, Strange RC, Fryer AA, Lear JT. Genetics and risk factors for basal cell carcinoma. Br J Dermatol. 2006;154(Suppl 1):5-7.
doi pubmed - Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31(2 Suppl 7):2-11.
doi pubmed - Torti DC, Christensen BC, Storm CA, Fortuny J, Perry AE, Zens MS, Stukel T, et al. Analgesic and nonsteroidal anti-inflammatory use in relation to nonmelanoma skin cancer: a population-based case-control study. J Am Acad Dermatol. 2011;65(2):304-312.
doi pubmed - Cahoon EK, Rajaraman P, Alexander BH, Doody MM, Linet MS, Freedman DM. Use of nonsteroidal anti-inflammatory drugs and risk of basal cell carcinoma in the United States Radiologic Technologists study. Int J Cancer. 2012;130(12):2939-2948.
doi pubmed - Clouser MC, Roe DJ, Foote JA, Harris RB. Effect of non-steroidal anti-inflammatory drugs on non-melanoma skin cancer incidence in the SKICAP-AK trial. Pharmacoepidemiol Drug Saf. 2009;18(4):276-283.
doi pubmed - Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sorensen HT. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer. 2012;118(19):4768-4776.
doi pubmed - Ma Y, Yu P, Lin S, Li Q, Fang Z, Huang Z. The association between nonsteroidal anti-inflammatory drugs and skin cancer: Different responses in American and European populations. Pharmacol Res. 2020;152:104499.
doi pubmed - Muranushi C, Olsen CM, Green AC, Pandeya N. Can oral nonsteroidal antiinflammatory drugs play a role in the prevention of basal cell carcinoma? A systematic review and metaanalysis. J Am Acad Dermatol. 2016;74(1):108-119.e101.
doi pubmed - Passarelli MN, Barry EL, Zhang D, Gangar P, Rees JR, Bresalier RS, McKeown-Eyssen G, et al. Risk of basal cell carcinoma in a randomized clinical trial of aspirin and folic acid for the prevention of colorectal adenomas. Br J Dermatol. 2018;179(2):337-344.
doi pubmed - Reinau D, Surber C, Jick SS, Meier CR. Nonsteroidal anti-inflammatory drugs and the risk of nonmelanoma skin cancer. Int J Cancer. 2015;137(1):144-153.
doi pubmed - Zhu Y, Cheng Y, Luo RC, Li AM. Aspirin for the primary prevention of skin cancer: A meta-analysis. Oncol Lett. 2015;9(3):1073-1080.
doi pubmed - Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, Williamson PR. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ. 2010;340:c365.
doi pubmed - Saini P, Loke YK, Gamble C, Altman DG, Williamson PR, Kirkham JJ. Selective reporting bias of harm outcomes within studies: findings from a cohort of systematic reviews. BMJ. 2014;349:g6501.
doi pubmed - Gordon LG, Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. Eur J Cancer Prev. 2015;24(2):141-149.
doi pubmed - Guy GP, Jr., Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48(2):183-187.
doi pubmed - Bains SJ, Mahic M, Myklebust TA, Smastuen MC, Yaqub S, Dorum LM, Bjornbeth BA, et al. Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study. J Clin Oncol. 2016;34(21):2501-2508.
doi pubmed - Suleiman S, Rex DK, Sonnenberg A. Chemoprevention of colorectal cancer by aspirin: a cost-effectiveness analysis. Gastroenterology. 2002;122(1):78-84.
doi pubmed - Swetter SM, Layton CJ, Johnson TM, Brooks KR, Miller DR, Geller AC. Gender differences in melanoma awareness and detection practices between middle-aged and older men with melanoma and their female spouses. Arch Dermatol. 2009;145(4):488-490.
doi pubmed - Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol. 2017;177(2):359-372.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


