| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 1, February 2023, pages 60-66
Estradiol Levels and Chemotherapy in Breast Cancer Patients: A Prospective Clinical Study
Widyanti Soewotoa, c , Nunik Agustrianib
aDivision of Surgical Oncology, Dr. Moewardi Hospital, Universitas Sebelas Maret, Surakarta, Indonesia
bDivision of Surgical Pediatric, Dr. Moewardi Hospital, Universitas Sebelas Maret, Surakarta, Indonesia
cCorresponding Author: Widyanti Soewoto, Division of Surgical Oncology, Dr Moewardi Hospital, Universitas Sebelas Maret, Surakarta, Indonesia
Manuscript submitted November 17, 2022, accepted December 31, 2022, published online February 26, 2023
Short title: Estradiol and Chemotherapy in Breast Cancer
doi: https://doi.org/10.14740/wjon1549
| Abstract | ▴Top |
Background: Breast cancer remains a global health issue, including in Indonesia, which has a relatively high incidence of breast cancer. Several theories have proved the role of estrogen in breast cancer carcinogenesis, but there is yet to be a preventive measure against breast cancer. Chemotherapy is one of the therapeutic modalities for breast cancer that disturbs ovarian function in producing estrogen due to damaged ovarian granulosa cells. Chemotherapy becomes an alternative option to decreasing circulating estradiol levels through interventions in ovarian functions, either by surgery, such as oophorectomy, or medications that disturb the ovarian functions. This study aimed to observe the estradiol levels in breast cancer patients before and after chemotherapy.
Methods: This was a prospective cohort study. We observed the estradiol levels before and after adjuvant chemotherapy in breast cancer patients. Subjects’ characteristics are presented in mean ± standard deviation, distribution frequency, and percentage. Subjects’ characteristics based on chemotherapy were tested using an independent t-test, Mann-Whitney U, and Chi-square/Fisher exact test. Effects of chemotherapy on estrogen levels were tested using the Wilcoxon rank test and Kruskal-Wallis test.
Results: A total of 194 research subjects were included. There were changes in estradiol levels before and after therapy. The decrease of estradiol levels in patients who did not receive chemotherapy was -6.9% (P > 0.05). Patients who received anthracycline cyclophosphamide (AC) regimen, paclitaxel and anthracycline (TA) regimen, paclitaxel, anthracycline and trastuzumab (TA + H) regimen, and platinum regimen experienced a significant decrease in estradiol levels (-21.4% (P < 0.05), -20.2% (P < 0.001), -31.7% (P < 0.01), and -23.7% (P < 0.05), respectively). Among chemotherapy groups, the estradiol levels before and after chemotherapy did not have significant differences (P = 0.937 and P = 0.730, respectively).
Conclusion: There are no significant differences in estradiol levels between chemotherapy and hormonal therapy groups. Patients in both groups have decreased estradiol levels after therapy, although patients in hormonal therapy do not experience as much decrease as those in chemotherapy.
Keywords: Estradiol levels; Chemotherapy; Breast cancer
| Introduction | ▴Top |
Breast cancer remains a global health issue, including in Indonesia, one of the Asian countries with a relatively high incidence of breast cancer. Data from The Indonesian Ministry of Health in 2018 showed that breast cancer was the most common type of cancer from 2015 to 2018 consecutively, with the total incidence and mortality rate continuing to rise to 42.1 per 100,000 population and 17 per 100,000 population, respectively [1].
Chemotherapy is one of the therapeutic modalities with several long-term side effects, such as cardiotoxicity, neurocognitive dysfunction, development of secondary malignancy, impaired kidney and liver functions, and gonadotoxicity. Ovarium, which produces estradiol, is highly sensitive to chemotherapy effects. Chemotherapy regimens, such as cyclophosphamide, busulfan, and 5-fluorouracil, have the highest number of premature ovarian failures. The regimens will impair ovarian functions and produce symptoms of menopause, osteoporosis, and infertility. However, the side effects of chemotherapy on ovarium, aside from causing detrimental effects on the reproductive function of premenopausal women, are also predicted to have therapeutic functions in decreasing estradiol levels [2].
Twenty to forty percent of women experience natural menopause, and serum estradiol (E2) levels will remain high for 6 - 12 months after menstruation stops. Therefore, amenorrhea during or after chemotherapy cannot be considered a cessation of the ovaries producing estradiol. In young women, the status of ovarian function cannot be determined from menstrual status unless they have undergone bilateral oophorectomy. The decrease in serum estradiol levels is a reference to the success of breast cancer therapy, by means of surgery such as oophorectomy or drugs that interfere with ovarian function [3-5].
The study’s objectives are to observe estradiol levels in breast cancer patients before and after adjuvant chemotherapy.
| Materials and Methods | ▴Top |
Study population
This was quantitative research in the form of prospective cohort observations at Dr. Moewardi Hospital between April 2021 and May 2022. This study observed changes in estradiol levels before and after adjuvant chemotherapy in breast cancer patients. The inclusion criteria were breast cancer patients who had undergone modified radical mastectomy or simple mastectomy, planned to undergo six cycles of chemotherapy, and had never received hormonal therapy or neoadjuvant chemotherapy. The exclusion criteria were comorbidities or conditions that could increase estradiol levels; patients lost to follow-up, namely, chemotherapy was not on schedule; incomplete chemotherapy; and death during chemotherapy.
Primary data collection included age, sex, and risk factors for breast cancer. Data on cancer included cancer stage, cell type, grade, subtype, and chemotherapy regimen. The estradiol levels were taken in the morning regarding the patient’s menopausal status, with normal values of 90 - 270 pg/mL using the DRG Estradiol ELISA, an immunoassay enzyme used for measuring the in vitro diagnostic quantity of estradiol in serum and plasma [6]. The estradiol levels were taken twice. The first was before the patient underwent the first cycle, and the second was 3 weeks after the patient went through the sixth cycle of chemotherapy.
Study design
Subjects’ characteristics are presented in mean ± standard deviation for numerical data and distribution frequency and percentage for categorical data. Subjects’ characteristics based on chemotherapy were tested using an independent t-test for normally distributed data, Mann-Whitney U for abnormally distributed and ordinal categorical data, and Chi-square/Fisher exact test for nominal categorical data. Effects of chemotherapy towards estrogen levels were tested using the Wilcoxon rank test (comparison of two means from paired samples) and Kruskal-Wallis test (comparison of more than two means from unpaired samples without normal distribution) (Table 1).
 Click to view | Table 1. Subjects’ Characteristics |
This study was approved by the Human Research Ethics Committee of Dr. Moewardi Hospital (ethical approval 41/I/HREC/2022). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Subjects’ characteristics
During 1 year of recruitment, 194 female patients with breast cancer met the inclusion and exclusion criteria. Subjects’ characteristics include age, age at menarche, cancer stage, grading, lymphovascular invasion (LVI), subtype, Ki67, menopausal status, marital status, number of children, breastfeeding, contraception, and family history.
The subjects’ characteristics showed that the mean age was 51.63 ± 10.01 years, and the mean age at menarche was 12.77 ± 1.41 years. Around half (51.5%) of the patients were categorized as locally advanced breast cancer (LABC). The cancer grading was dominated by grade III (60.3%) and LVI positive (68.6%). The most common and least subtypes were human epidermal growth factor receptor 2 (HER2) (25.8%) and triple-negative (10.8%), respectively. Ki67 were mostly negative (57.2%). Women in menopause (42.3%) were fewer than premenopause (57.7%). Almost all patients (95.4%) were married, and the majority (57.2%) had 1 - 2 kids. More than three-quarters (78.4%) had a history of breastfeeding. Patients with hormonal contraception (38.1%) outnumbered those with nonhormonal contraception (29.4%). Most patients (82%) had no family history of cancer.
Statistical analysis
In Table 2, based on the cancer subtype, estradiol levels decreased after chemotherapy compared to before.
 Click to view | Table 2. Estradiol Levels Before and After Therapy Based on Subtypes |
Effects of chemotherapy on estrogen levels in breast cancer patients
Effects of chemotherapy on estrogen levels in breast cancer patients showed decreased estrogen levels based on chemotherapy regimens. Table 3 shows that among all groups, patients who did not receive chemotherapy had an insignificant decrease (P > 0.05) in mean estradiol levels after therapy (-6.9%; P = 0.163). Meanwhile, all chemotherapy regimens produced significantly decreased estradiol levels (P < 0.05) after therapy, with the paclitaxel, anthracycline and trastuzumab (TA + H) regimen bringing the highest changes (-31.7%), followed by the platinum regimen (-23.7%), anthracycline cyclophosphamide (AC) regimen (-21.4%), and paclitaxel and anthracycline (TA) regimen (-20.2%).
 Click to view | Table 3. Effects of Chemotherapy on Estrogen Levels |
Between patients who received chemotherapy and those who did not, there were no significant differences (P > 0.05) in estradiol levels before and after therapy (P = 0.937 and P = 0.730, respectively).
| Discussion | ▴Top |
Estradiol is a hormone naturally produced in the human body by the ovaries, placenta, testes and in small amounts by the adrenal cortex. The normal functions of estradiol are regulating the menstrual cycle, cardiovascular system, neurological system, skeletal system, vascular system, and many more. Estradiol is the most potent and abundant estrogen (E2) during reproductive years in women. Estradiol binds to specific intracellular estrogen receptors in female reproductive organs, breasts, hypothalamus, and hypophysis. The receptor-ligand complex promotes gene expression for maintaining fertility and secondary sexual characteristics in women. In addition, estradiol shows anabolic features, slow metabolism, and increased blood coagulability. Estradiol, the primary intracellular estrogen in humans, is substantially more active at the cellular level than its metabolites, estrone and estriol [7].
Chemotherapy has different effects on ovarian tissue depending on the patient’s age, chemotherapy regimen, therapy dosage and duration. Several anticancer drugs cause direct damage to the oocytes, slower maturation, destruction and reduction of the follicle pool, cortical fibrosis, vascular damage, and ovarian atrophy [8].
In this study, 17 (8.8%) patients did not receive chemotherapy. The numbers of patients who received the AC and TA regimens were 42 (21.6%) and 91 (46.9%), respectively. The TA + H regimen and platinum regimen each consisted of 22 (11.3%) patients.
Table 1 shows no significant differences (P > 0.05) between patients who received chemotherapy and those who did not, based on the following risk factors for breast cancer: age (P = 0.946), age at menarche (P = 0.793), cancer grading (P = 0.332), LVI (P = 0.366), Ki67 (P = 0.888), menopausal status (P = 0.675), marital status (P = 0.570), number of kids (P = 0.959), breastfeeding (P = 0.128), contraception use (P = 0.312), and family history of cancer (P = 0.318). Hence, chemotherapy was not influenced by these risk factors. Meanwhile, there were significant differences (P < 0.05) based on cancer staging (P = 0.033) and subtype (P = 0.004).
In addition, patients who received chemotherapy were most frequently categorized as LABC (52.0%), followed by ABC (28.2%) and EBC (19.8%). HER2 subtype was the most frequent subtype in patients who received chemotherapy, followed by luminal B (20.9%), luminal A (20.3%), triple-negative (19.2%), and triple-positive (10.8%).
The chemotherapy regimens in this study used a combination of anthracycline 60 mg/m2, cyclophosphamide 600 mg/m2 (AC) for early-stage cancer and a combination of anthracycline 600 mg/m2 and paclitaxel 175 mg/m2 (TA) for late-stage cancer. Most patients with the triple-negative subtype were given a platinum carboplatin area under the curve (AUC) 6 regimen. In the triple-positive subtype, the regimens were given according to cancer stage in addition to trastuzumab 8 mg/kg IV day 1 followed by 6 mg/kg IV (TA + H) [9].
Chemotherapy causes significant cytotoxic effects on live cells. This mechanism correlates with the risk of ovarian failure and infertility due to chemotherapy. Alkylating antineoplastic drugs (cyclophosphamide, mechlorethamine, chlorambucil) bind to the target cell’s DNA, causing the inhibition of DNA synthesis and functions. This process causes DNA chain breaking and apoptosis mediated by P63 on primordial follicles. The use of alkylating antineoplastic agents is accompanied by the highest risk of infertility. Anthracyclines (doxorubicin, bleomycin, mitomycin, epirubicin) inhibit DNA synthesis and functions and disrupt DNA transcription. The inhibition of topoisomerase II causes DNA damage, which leads to toxic free radical oxygen formation that results in DNA chain breaking and inhibition of DNA synthesis and functions. Anthracycline has rare gonadotoxic effects and a low-to-moderate risk of infertility. A study by Zhen et al on 136 breast cancer patients found that estrone (E1), estradiol (E2), and follicle-stimulating hormone (FSH) levels decreased after chemotherapy with anthracycline. The decrease in tumor size in the anthracycline group was also larger than those in the cyclophosphamide, methotrexate and fluorouracil (CMF) group [10-13].
In this study, patients received chemotherapy according to their cancer stage and subtype (Fig. 1). Chemotherapy was shown to decrease estradiol levels, and in this study, it was seen that estradiol levels before and after treatment, the triple-positive subtype seemed to cause a decrease in estradiol levels more than other subtypes.
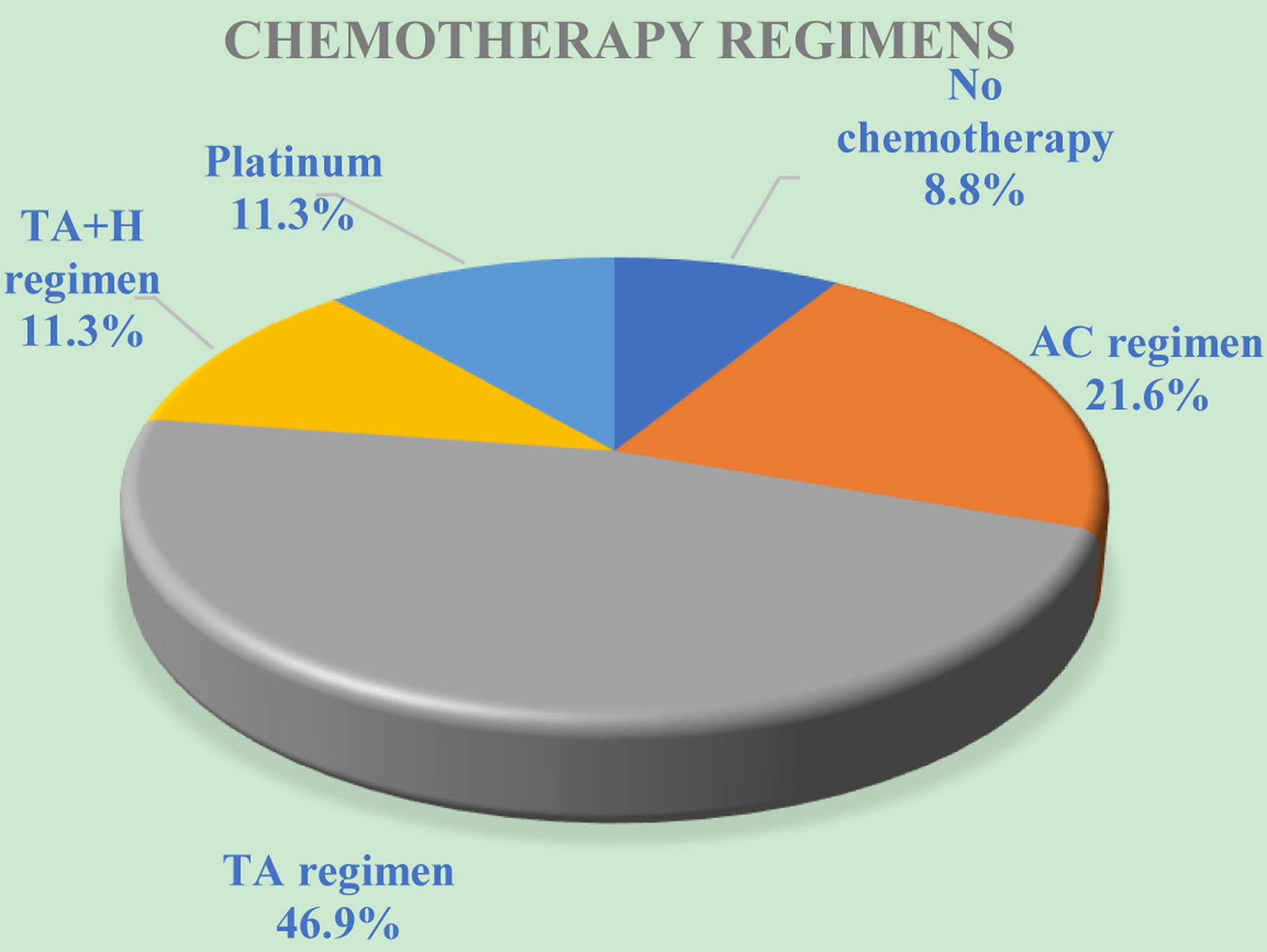 Click for large image | Figure 1. The distribution of breast cancer patients based on chemotherapy regimens. AC: anthracycline cyclophosphamide. TA: paclitaxel, anthracycline. TA + H: paclitaxel, anthracycline and trastuzumab. |
Patients who received the AC regimen experienced a 21.4% decrease in estradiol levels after chemotherapy (P = 0.011), whereas patients who received the TA regimen experienced a 20.0% decrease. Patients with TA + H combined regimen showed the highest and statistically significant (P < 0.05) decrease of 31.7% in estradiol levels.
Out of 21 breast cancer patients who had HER2 overexpression, seven were premenopausal, and 14 were postmenopausal. Out of seven premenopausal patients who had high serum estradiol levels before chemotherapy, two had increased estradiol levels, and five had decreased estradiol levels after therapy. Although they experienced a decrease, four still had high estradiol levels, and one had normal estradiol levels. Meanwhile, out of 14 postmenopausal patients, four had an increase, and 10 had a decrease in estradiol levels after chemotherapy, but all remained having high estradiol levels [14].
Platinum preparations (cisplatin, carboplatin) covalently bind to DNA, forming intra-chain DNA cross-links. This causes the DNA to rupture during replication, inhibiting transcription and disrupting DNA synthesis and function. The use of platinum drugs is accompanied by a moderate risk of infertility. In this study, patients who received the platinum regimen had a statistically significant decrease of -23.7% (P = 0.016) in estradiol levels after chemotherapy. A study by Septiani et al [15] in 2022 revealed that 91.3% of patients with triple-negative subtype had a mean decrease of 11.57 pg/mL in estradiol levels after therapy, whereas the rest (8.7%) had a mean increase of 16.5 pg/mL (P = 0.001).
In patients who did not undergo chemotherapy, patients received hormonal therapy, and evaluation of estradiol levels during six cycles of hormone therapy showed a statistically insignificant decrease (P > 0.05) of 6.9% (P = 0.163) after therapy.
It was reported that many hormones, such as E1, E2, and FSH, can increase the proliferation and invasion of cancer cells in women. The mechanism may be related to the activation of proteins, enzymes, or oncogenes involved in nucleic acid synthesis induced by these hormones, thereby promoting the growth of cancer cells. However, in the study from Huang et al, it was found that high serum E2 levels were associated with reduced risk of drug-induced liver injury (DILI) in premenopausal breast cancer patients who underwent epirubicin regimen chemotherapy with cyclophosphamide adjuvant chemotherapy [16, 17].
Between patients who received chemotherapy and those who did not, there were no significant differences (P > 0.05) in estradiol levels before and after therapy (P = 0.937 and P = 0.730, respectively). However, patients who received chemotherapy experienced a greater decrease in estradiol levels after therapy than they did not receive chemotherapy and received only hormone therapy.
The limitation of this study was limited observations, as we only observed the estradiol levels 3 weeks after chemotherapy. Hence, we could not conclude if the estradiol levels would stay low or increase to the levels before chemotherapy. A study by Shamrai et al on 26 breast cancer patients showed a significant decrease in estradiol levels throughout a 3-year observation [18].
Conclusion
There are no significant differences in estradiol levels between chemotherapy and hormonal therapy. Estradiol levels decreased after therapy in both groups, although patients in hormonal therapy do not experience as much decrease as those in chemotherapy.
Acknowledgments
The authors would like to thank Dr. Moewardi General Hospital as allowing permission to do this case report.
Financial Disclosure
The authors received financial support for the research of this article from the University’s non-budgetary fund of state revenue and expenditure in March 2022, with the number 254/UN27.22/PT01.30/2022.
Conflict of Interest
The authors declare no potential conflict of interest.
Informed Consent
Informed consent was obtained from the subject of the study for participation in the research and publication of the results.
Author Contributions
Conceptualization: WS and NA. Methodology: WS. Data curation: WS. Formal analysis: WS and NA. Original draft preparation: WS. Writing-review and editing: WS. All authors have contributed equally, read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kemenkes RI. Pusat Data dan Informasi Kementerian Kesehatan Republik Indonesia. 2015.
- Yang B, Shi W, Yang J, Liu H, Zhao H, Li X, Jiao S. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a meta-analysis of randomized controlled trials. Breast. 2013;22(2):150-157.
doi pubmed - Braverman AS, Sawhney H, Tendler A, Patel N, Rao S, El-Tamer M, Kamenova B, et al. Serum estradiol above the postmenopausal level after chemotherapy-induced amenorrhea in breast cancer patients. Therapy. 2006;3(5):609-612.
doi - Vriens IJ, De Bie AJ, Aarts MJ, de Boer M, van Hellemond IE, Roijen JH, van Golde RJ, et al. The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget. 2017;8(7):11372-11379.
doi pubmed - Chen H, Xiao L, Li J, Cui L, Huang W. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2019;3(3):CD008018.
doi pubmed - DRG Estradiol ELISA (EIA-2693). 2010. DRG International, Inc. USA.
- Hariri L, Rehman A. Estradiol. In: StatPearls. Treasure Island (FL). 2022.
- Zhao Y, Wang X, Liu Y, Wang HY, Xiang J. The effects of estrogen on targeted cancer therapy drugs. Pharmacol Res. 2022;177:106131.
doi pubmed - NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. 2022. Version 4.2022. June 21, 2022. NCCN Guidelines for Patients® available at: www.nccn.org/patients.
- Amjad MT, Chidharla A, Kasi A. Cancer chemotherapy. In: StatPearls. Treasure Island (FL). 2022.
- Zhen H, Guo F, Zhang X, Jia M, Yang H, Yao Y, et al. The effects of chemotherapy with anthracyclines vs capecitabine on tumour size, survival rate and estradiol levels in patients with locally advanced breast cancer. Eur J Gynaecol Oncol. 2020;41(5):785-789.
doi - Zhen H, Fan G, Xiaojun Z, Jia M, Haibo Y, Yarong Y, et al. Anthracycline chemotherapy in treating advanced breast cancer and its effect on estradiol and tumor size. Indian J Pharm Sci. 2020;82(1):124-128.
doi - Abotaleb M, Kubatka P, Caprnda M, Varghese E, Zolakova B, Zubor P, Opatrilova R, et al. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed Pharmacother. 2018;101:458-477.
doi pubmed - Zaini IH, Soewoto W, Budhi IB. The effect of chemotherapy on estradiol levels in patients with HER 2-overexpression breast cancer in Dr Moewardi General Hospital, Surakarta, Indonesia. Open Access Macedonian Journal of Medical Sciences. 2021;9(B):1570-1574.
doi - Septiani RV, Soewoto W, Budhi IB. Chemotherapy effect on estradiol levels in patients with triple-negative breast cancer: a clinical prospective study from Indonesia. Open Access Macedonian Journal of Medical Sciences. 2022;10(B):477-481.
doi - Huang S, Liu M, Fu F, Liu H, He B, Xiao D, Yang J. High serum estradiol reduces acute hepatotoxicity risk induced by epirubicin plus cyclophosphamide chemotherapy in premenopausal women with breast cancer. Front Pharmacol. 2020;11:572444.
doi pubmed - Malisic E, Susnjar S, Milovanovic J, Todorovic-Rakovic N, Kesic V. Assessment of ovarian function after chemotherapy in women with early and locally advanced breast cancer from Serbia. Arch Gynecol Obstet. 2018;297(2):495-503.
doi pubmed - Shamrai VA, Misiurko OI, Grebeniuk DI. Dynamics of changes in the main indicators of reproductive health of women receiving chemotherapy for malignant breast tumors. Biomed Biosoc Anthropol. 2021;(43):5-12.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


