| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 1, February 2023, pages 40-50
Real-World Experience of Adults With Acute Myeloid Leukemia on Hypomethylating Agents With or Without Venetoclax at a Comprehensive Cancer Center
Tracelyn Freemana, d, Kiersten Williamsa, Marcin Putoa, Allyson Wallera, Eric M. McLaughlinb, James S. Blachlyc, Julianna Roddya
aDepartment of Pharmacy, The Ohio State University, Columbus, OH, USA
bDepartment of Biomedical Informatics, The Ohio State University, Columbus, OH, USA
cDepartment of Hematology, The Ohio State University, Columbus, OH, USA
dCorresponding Author: Tracelyn Freeman, The James Cancer Hospital at The Ohio State University, Columbus, OH 43212, USA
Manuscript submitted December 15, 2022, accepted January 9, 2023, published online February 26, 2023
Short title: Hypomethylating Agents and Venetoclax in AML
doi: https://doi.org/10.14740/wjon1557
| Abstract | ▴Top |
Background: Venetoclax (VEN) in combination with hypomethylating agent (HMA) therapy is a standard treatment option for patients with newly diagnosed acute myeloid leukemia (AML); however, data are limited in the relapsed or refractory (R/R) populations and in those with poor-risk disease. A retrospective review was conducted involving patients with AML who received HMA alone or in combination with VEN (VEN + HMA).
Methods: VEN + HMA was compared to HMA alone in first-line and R/R settings. Patients were stratified by specific HMA and line of therapy. The primary endpoint was overall response rate (ORR) up to 6 months from start of treatment.
Results: Fifty-two patients were evaluated for efficacy and 78 patients for safety. ORR was 67% (VEN + HMA) versus 80% (HMA) in the first line and 50% versus 22% in R/R setting. A greater clinical benefit was seen with VEN + HMA compared to HMA in both lines of therapy (first-line: 87% vs. 80%; R/R: 75% vs. 67%). The median duration of response was longer with VEN + HMA first-line, but shorter in the R/R setting compared to HMA (8.3 vs. 7.2 months and 2.5 vs. 3.7 months, respectively). Of the 32 patients who responded to therapy, 63% had a complex karyotype. Survival benefits were greater with VEN + HMA in both lines of therapy, although not statistically significant. Grade 3/4 neutropenia was reported in all patients receiving VEN, and 95% of these patients also experienced grade 3/4 thrombocytopenia. There were three cases of tumor lysis syndrome.
Conclusion: The addition of VEN to HMA has consistently shown benefit as first-line treatment and may have some benefit in R/R settings as well. Further studies are needed to compare across various lines of treatment and unfavorable disease. Dynamic strategies that improve toxicity management should be considered.
Keywords: Venetoclax; Acute myeloid leukemia; Hypomethylating agents; Less intensive; Relapsed; Unfavorable
| Introduction | ▴Top |
Acute myeloid leukemia (AML) carries the highest incidence among adult leukemias in the United States, with approximately 20,000 new cases per year. Clinical outcomes of AML vary given the heterogeneity of this disease and although they have been overall poor, more recently the advancements in genetic testing have allowed for more individualized therapy [1]. Historically, the backbone of treatment consisted of an anthracycline and cytarabine combination for induction with transplant evaluation in select cases; however, the majority of older adults are unable to tolerate these intense regimens and may have a poorer prognosis [2].
Prior to the advent of targeted therapies, hypomethylating agents (HMAs) or low-dose cytarabine were treatment options that appeared more tolerable but yielded low overall response rates (ORRs, less than 50%) in newly diagnosed and relapsed or refractory (R/R) populations [3, 4]. Advancements in targeted therapy against FLT3 and IDH mutations now provide a single-agent oral option for newly diagnosed or relapsed disease that express these biomarkers [5-8], but in the absence of these mutations, patients faced few options for less intensive treatment.
A new era has introduced the combination of venetoclax (VEN) with HMA or low-dose cytarabine which is approved for the treatment of newly diagnosed AML in patients aged 75 or older, and in patients with comorbidities that preclude the use of intensive chemotherapy. VEN targets the anti-apoptotic protein BCL2 which is expressed on many hematological cancer cells, and this inhibition restores normal apoptosis in leukemia cells leading to destruction [9, 10]. The advent of VEN and the expansion of targeted therapy has changed the landscape for AML treatment, particularly in patients who are not candidates for intensive induction.
Early phase approval and confirmatory studies, including the phase 1b dose escalation/expansion study of VEN combined with azacitidine or decitabine and the phase 2 study of 10-day decitabine in combination with VEN, had promising results and led to the increased use of VEN in combination with HMA beyond the first-line setting [11-13]. This use in practice may now capture patients with R/R disease who are not candidates for more intensive salvage chemotherapy. Further, the use of the VEN combination beyond the untreated elderly population is desirable as it may provide improved outcomes without the additional toxicity seen with standard intensive chemotherapy.
VEN now provides an additional lower intensity treatment option, particularly in patients without actionable mutations; however, comparison of HMA with VEN to HMA alone in the first-line setting is a newer addition to the literature, leaving an unmet need in R/R populations and those with unfavorable risk disease. Furthermore, it is not yet understood how treatment should be adjusted for severe hematological toxicities seen in practice.
Therefore, in this study we sought to investigate the clinical and safety outcomes of patients with AML who received the combination of VEN and HMA (VEN + HMA) compared to those who received HMA alone in a single-institution historical control cohort.
| Materials and Methods | ▴Top |
Study design and inclusion/exclusion criteria
This is a single-center, retrospective cohort review that included patients aged 18 years or greater with a diagnosis of AML that received azacitidine or decitabine with or without VEN between June 1, 2017 and December 5, 2019 at The Ohio State University Comprehensive Cancer Center - The James Cancer Hospital. To be eligible for inclusion in the efficacy analysis, patients had to receive either 28 days of VEN or reach cycle 2, day 1 of azacitidine or decitabine. To be eligible for inclusion in the safety analysis, patients had to receive at least one dose of either azacitidine or decitabine and VEN, if applicable. Protected populations including pregnant or imprisoned patients, and patients enrolled on a clinical trial were excluded. This study protocol was approved by the Institutional Review Board. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Definitions
Complete remission (CR) was defined as the morphological recovery evidenced by less than 5% blasts according to bone marrow biopsy with complete hematological recovery. CR with incomplete count recovery (CRi) was defined as the morphological recovery evidenced by less than 5% blasts according to bone marrow biopsy, but with absolute neutrophil count (ANC) ≤ 1,000/µL or platelets ≤ 100,000/µL. ORR was comprised of CR and CRi. Morphological leukemia-free state (MLFS) was defined as morphological recovery without hematological recovery (ANC ≤ 1,000/µL and platelets ≤ 100,000/µL) [14]. Transfusion independence was defined as any 8-week period where the patient did not require blood or platelet transfusions. Clinical benefit was characterized by achievement of response (CR, CRi, or MLFS) or transfusion independence.
Study endpoints
The primary endpoint of this study was the ORR (CR or CRi) following receipt of HMA with or without VEN up to 6 months from the start of treatment. Response rates were evaluated by the research team and retrospectively validated by physician review. Secondary objectives included clinical benefit rate, time to first response, time to treatment failure, overall survival (OS), transfusion independence, correlations between genomic aberrations and response to therapy, and safety, including incidence and severity of adverse effects. Toxicity as documented by the treating physician was determined by review of physicians’ notes and recorded laboratory values. Toxicities studied were grade 3/4 neutropenia, grade 3/4 thrombocytopenia, grade 3/4 changes in serum creatinine, and laboratory signs of tumor lysis syndrome (TLS), and were graded according to Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 [15].
Statistical analysis
As an exploratory study, the primary aim of the analysis was to present descriptive estimates of the CR rates for both treatment groups. Secondary outcomes including the ORR, the clinical benefit rate, OS, time to response, time to treatment failure, and adverse events were also analyzed. Patients with incomplete medical records were not a part of the analysis.
All data were collected utilizing Research Electronic Data Capture (REDCap®) [16]. Patient demographics and outcomes were reported for each treatment group and compared using Fisher’s exact test or two-sample t-tests, as appropriate. Efficacy outcomes (CR rate, ORR, and clinical benefit rate) were compared between groups for patients treated with 28 days of VEN and reached cycle 2, day 1 of azacitidine or decitabine. Differences in response to therapy were also compared by the presence or absence of various genetic aberrations. Kaplan-Meier curves were generated for each treatment group for OS, time to response and time to treatment failure. Patients were censored at the date of their last physician visit.
Safety outcomes were reported for all patients who have received at least one dose of VEN, azacitidine or decitabine including frequencies of toxicities, adverse events, and any dose delays or reductions. These outcomes were compared between treatment groups using Fisher’s exact tests. Duration of neutropenia was compared using the Wilcoxon rank-sum test. As a sensitivity analysis, response to therapy was also analyzed, including patients who did not meet the required minimum therapy but had at least one dose of VEN, azacitidine or decitabine. P-values reported are for descriptive purposes, and given the exploratory nature of the study, strong conclusions should not be inferred from the results of the statistical tests. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
| Results | ▴Top |
Patient screening
Out of 103 patients screened for inclusion, 26 patients were excluded. Reasons for exclusion included receipt of HMA with an agent other than VEN or involvement on a clinical trial. Thus, a total of 77 patients were included in the study. Treatment groups for the entire population included 38 patients in the HMA group and 39 patients in the VEN + HMA group (Fig. 1).
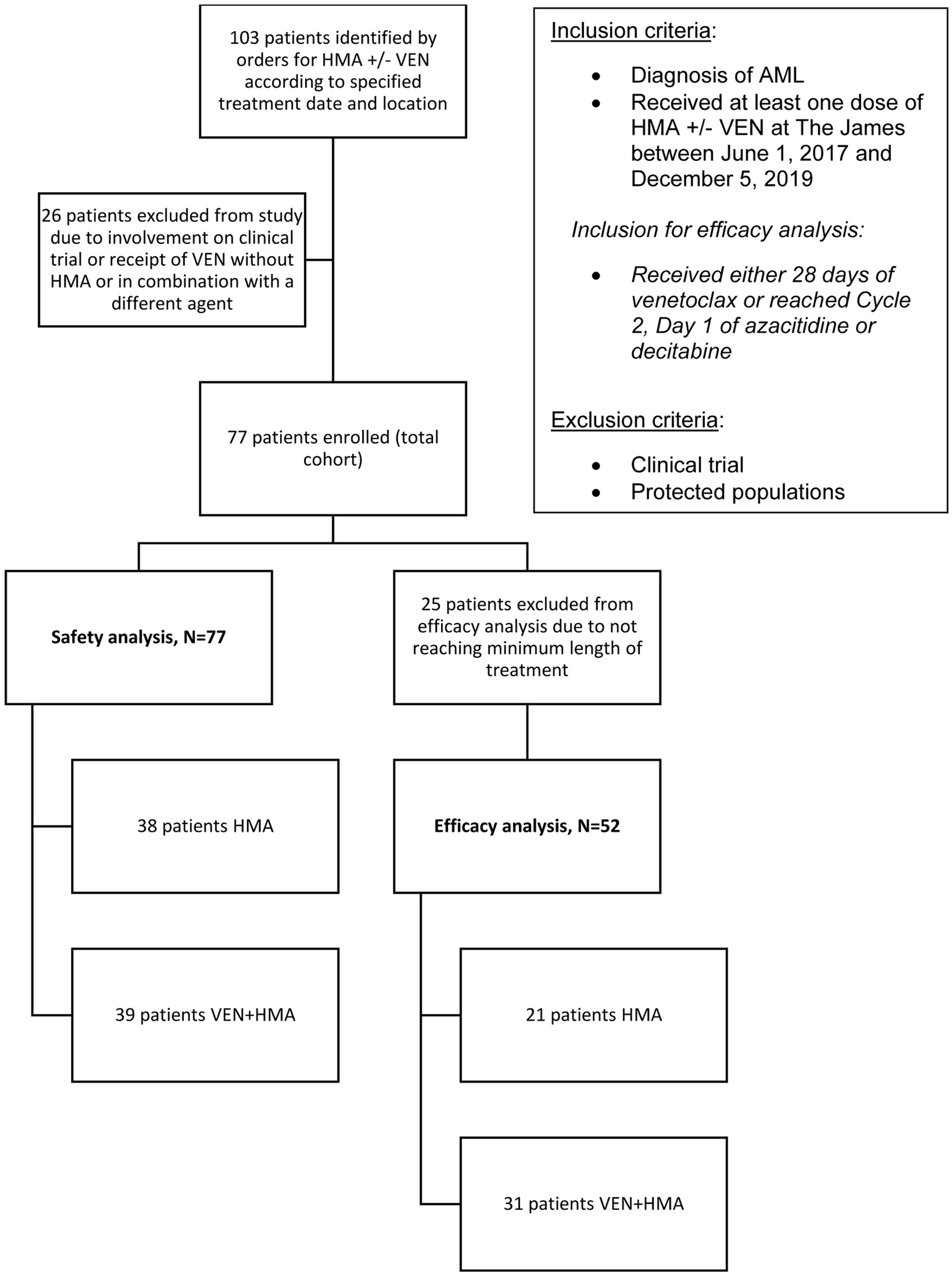 Click for large image | Figure 1. The selection process for study enrollment. Records were identified by electronic orders according to treatment date and location criteria. Diagnosis and treatment were verified creating the total cohort, all of which were included in the safety analysis. The efficacy analysis was comprised of patients that received the minimum length of treatment as specified. |
Patient demographics
Baseline characteristics are shown in Table 1 and were overall similar between groups. The median age in the HMA group was 64 years and the median age in the VEN + HMA group was 68 years. For the HMA alone group, 92% of patients received decitabine compared to only 8% of patients that received azacitidine. The use of azacitidine and decitabine was more evenly distributed in the VEN + HMA group. Prior HMA therapy was reported in 18.4% of patients in the HMA group compared to 25.6% of patients in VEN + HMA group. All but two patients were started on antifungal prophylaxis with VEN beginning cycle 1 day 1, with posaconazole and fluconazole being the most frequently prescribed agents. In most cases, the VEN ramp-up was prescribed according to the current FDA-approved labelling for VEN. The ramp-up and target dose aligned with the prescribed antifungal for 24 patients, whereas six patients were ramped up according to a different schedule. Only one patient did not receive a ramp-up for VEN.
 Click to view | Table 1. Baseline Characteristics |
Efficacy
A total of 21 patients in the HMA group and 31 patients in the VEN + HMA group were included in the efficacy analysis. The response rates for first-line and R/R settings are shown in Table 2, with a higher ORR seen with the VEN + HMA group in the R/R setting. ORR was lower with VEN + HMA compared to HMA in the first-line setting (67% vs. 80%) but with a sample triple the size of HMA (n = 15 vs. n = 5). The rate of MLFS was higher with VEN in the first-line setting, but lower with VEN in the R/R setting. A higher number of patients who received VEN + HMA compared to HMA achieved transfusion independence (45% vs. 14%), and this trend was consistently seen across first-line and R/R settings. The clinical benefit according to response in the total efficacy cohort was 82% vs. 71% with VEN + HMA and HMA, respectively, which further breaks down to 87% vs. 80% in the first-line, and 75% vs. 67% in the R/R setting.
 Click to view | Table 2. Response Rates |
In the total cohort, the median time to response was longer with VEN + HMA compared to HMA alone in the first-line setting (1.9 vs. 1.3 months). The median duration of response was longer with VEN + HMA first-line, but shorter in the R/R setting compared to HMA (8.3 vs. 7.2 months) and (2.5 vs. 3.7 months), respectively. A total of five patients subsequently received a bone marrow transplant, all of which were in the HMA group.
Risk stratification
Of the 32 patients who responded to therapy, 63% had a complex karyotype and the most commonly reported aberrations were located at ASXL1, RUNX1, TET2, and TP53. Genomic abnormalities were characterized in patients who received clinical benefit (CR/CRi/MLFS) and are shown in Table 3, with 22 patients with VEN + HMA and 10 patients with HMA alone achieving benefit. A similar proportion of responding patients for each treatment were classified as poor-risk disease (VEN + HMA: 82% vs. HMA alone: 80%). In responding patients, a greater proportion of patients in the HMA group had a complex karyotype compared to VEN + HMA patients (70% vs. 59%).
 Click to view | Table 3. Comparisons of Risk Stratification by Treatment in Benefiting Patients (CR/CRi/MLFS) |
Survival
In the first-line and R/R settings, the median progression-free survival (PFS) was slightly longer with VEN + HMA compared to HMA alone (median 8.9 vs. 7.3 months and median 4.3 vs. 3.0 months, respectively). Regarding OS, it appeared that a bigger difference was seen with VEN + HMA in both the first-line and R/R setting (median 11.0 vs. 7.3 months and median 8.4 vs. 4.9 months, respectively) (Fig. 2).
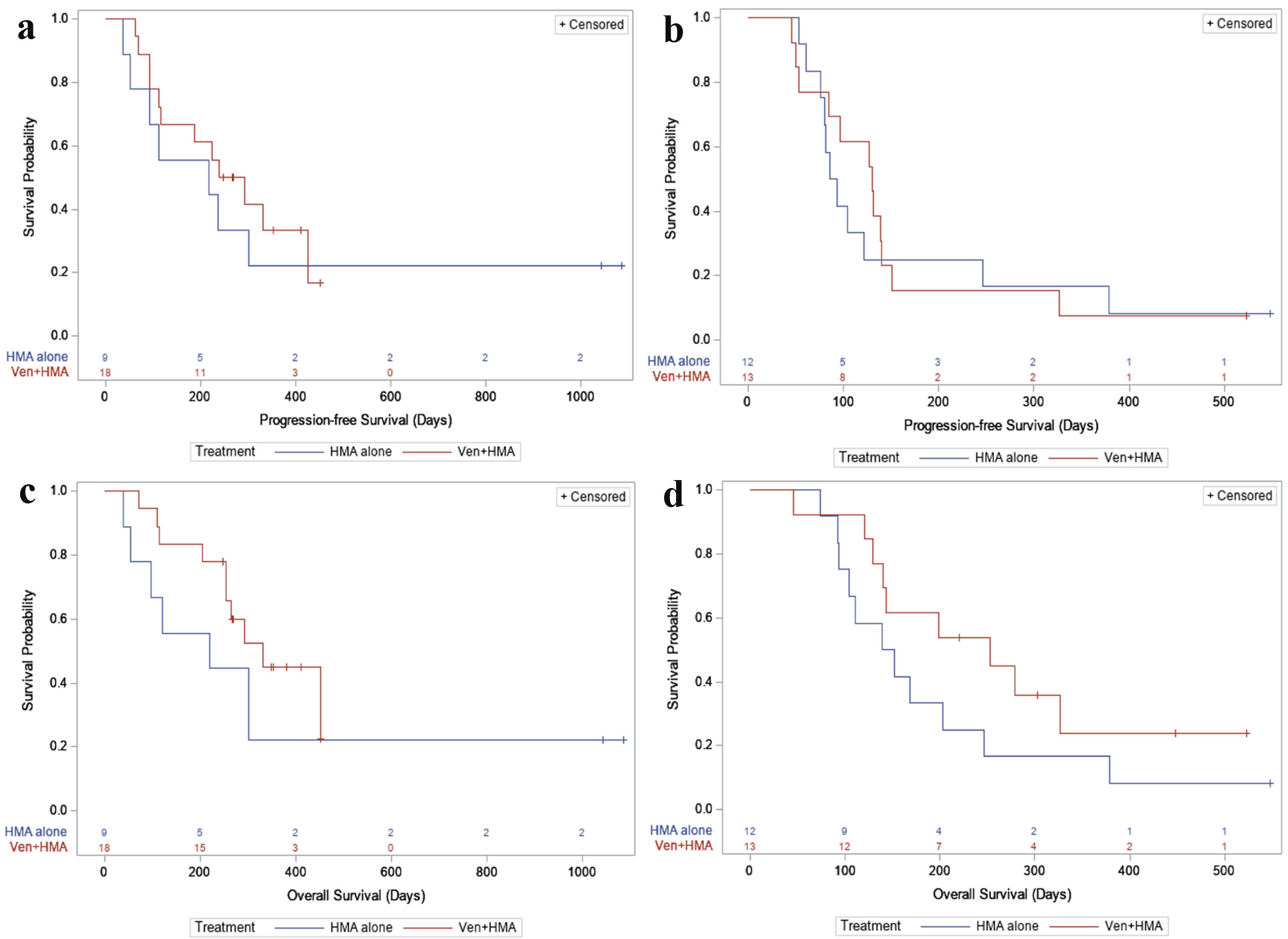 Click for large image | Figure 2. (a) PFS, first-line. For newly diagnosed patients: in the HMA alone group, 7/9 (77.8%) had a PFS event during follow-up, with a median PFS time of 218 days (95% CI: 36 - not reached). In the VEN + HMA group, 12/18 (66.7%) had a PFS event, with a median PFS time of 266 days (95% CI: 112 - 425). (b) PFS, relapsed or refractory. For relapsed/refractory patients: in the HMA alone group, 11/12 (91.7%) had a PFS event during follow-up, with a median PFS time of 89.5 days (95% CI: 61 - 246). In the VEN + HMA group, 12/13 (92.3%) had a PFS event, with a median PFS time of 130 days (95% CI: 53 - 140). (c) OS, first-line. In newly diagnosed patients: in the HMA alone group, 7/9 (77.8%) patients died during follow-up, with a median survival of 219 days (95% CI: 39 - not reached). In the VEN + HMA group, 10/18 (55.6%) patients died, with a median survival of 331 days (95% CI: 253 - not reached). (d) OS, relapsed or refractory. In relapsed/refractory patients: in the HMA alone group, 11/12 (91.7%) patients died during follow-up, with a median survival of 145.5 days (95% CI: 92 - 246). In the VEN + HMA group, 9/13 (69.2%) patients died, with a median survival of 253 days (95% CI: 129 - not reached). VEN: venetoclax; HMA: hypomethylating agent; PFS: progression-free survival; OS: overall survival; CI: confidence interval. |
Safety
Toxicities are shown in Table 4. A dose delay at any point in therapy was reported in 34% of patients in the HMA group and 68% of patients with VEN + HMA. The incidence of HMA-specific dose reductions was twice as high in the VEN + HMA group and 38% of patients had a VEN-specific dose reduction. Grade 3/4 neutropenia occurred in 76% of patients with HMA and 100% of patients with VEN + HMA. Profound neutropenia, defined as ANC < 100/µL, was reported in 74.3% of patients who received VEN + HMA. Grade 3/4 thrombocytopenia occurred in most patients in either group.
 Click to view | Table 4. Toxicity |
A total of 20 patients received cycle 1 treatment while hospitalized. Of the 58 non-hospitalized patients, 19 (33%) required hospitalization due to infection and 23 (40%) required hospitalization due to febrile neutropenia. Comparing hospitalizations between VEN + HMA and HMA, this further breaks down to 41% vs. 24% for suspected infection, and 38% vs. 41% for febrile neutropenia, respectively. There were three cases of TLS reported with VEN. All three cases received allopurinol prophylaxis and required intervention including hydration, rasburicase, and phosphate binders. One required renal replacement therapy.
| Discussion | ▴Top |
Initial approval for VEN was based on the results of a phase 1b dose escalation and expansion study in which patients received VEN in combination with decitabine or azacitidine. Out of 145 newly diagnosed patients with a median age of 74 years, 67% of enrolled patients achieved a CR including those with incomplete count recovery. Patients with poor-risk cytogenetics achieved a complete response of 60%. Most common adverse effects included grade 3/4 hematological toxicities and grade 1/2 gastrointestinal toxicities [11]. Moreover, the confirmatory phase 3 multicenter, randomized, double-blind, placebo-controlled trial of 431 patients produced consistent results with a composite CR of 67% further supporting the addition of VEN to azacitidine for newly diagnosed AML [12].
Another study conducted by DiNardo et al investigated the combination of VEN with HMA beyond the first-line setting. A single-center, phase 2 study at the University of Texas MD Anderson Cancer Center enrolled 168 patients that evaluated the induction regimen of 10-day decitabine and VEN in first and subsequent lines of therapy for AML including untreated and previously treated secondary AML and R/R AML. Following induction, decitabine was reduced to 5 days for consolidation. The ORR was 74% with response rates ranging from 61% to almost 90% in the various disease subgroups. Unsurprisingly, the common adverse events were infection-related, grade 3 or 4 neutropenia, and febrile neutropenia [13].
Expectedly, patients showed response to VEN + HMA in our study. For newly diagnosed patients with AML, the combined CR/CRi rate was numerically lower with VEN + HMA compared to HMA (67% vs. 80%) which may appear surprising initially. Although not consistent with the findings from the reference DiNardo study, the percent CR/CRi was similar to the 67% reported in their original article, and our study also included a higher number of high-risk patients (82% vs. 25%). The lower rates seen with VEN + HMA frontline in our study might be explained by a substantially larger sample size compared to HMA (VEN + HMA = 15 vs. HMA = 5). Above all, we do not feel this finding supports use of HMA alone over VEN + HMA, especially since when looking at CR only and the clinical benefit rate, VEN + HMA appeared to achieve a better response.
Until recently, the combination of VEN + HMA had only been evaluated in patients with newly diagnosed AML. Although it is supported in the National Comprehensive Cancer Network (NCCN) guidelines, there has yet to be a second FDA approval for this indication. This study adds to the literature by including patients with R/R disease and describes response to VEN + HMA in a real-world setting. Furthermore, Figure 2c and d suggests there may be an OS benefit in both lines of therapy, but larger studies are needed for a stronger statistical analysis with greater representation of favorable-risk NPM1-mutated disease to investigate any improvement in survival.
Compared to HMA, VEN + HMA demonstrated a longer PFS, but a more pronounced separation was seen with OS. It is important to recognize that even though VEN + HMA therapy likely achieves a deeper remission, patients are still at risk for relapse. These patients may live longer allowing the opportunity to receive other treatments including low-dose cytarabine or a targeted agent subsequently. Duration of response was longer with VEN + HMA in the first-line setting, but was shorter in the R/R setting which could partly be due to the increased toxicities seen with VEN coupled with heavily pre-treated disease. Most notably, severe neutropenia led to frequent dose delays and reductions in some patients with multiple therapy interruptions ultimately contributing to treatment failure.
Surprisingly, the median time to response was longer with VEN + HMA compared to HMA alone (1.9 vs. 1.3 months). This could be due to a difference in timing of the bone marrow assessment, as we typically perform a biopsy after the first cycle of VEN + HMA; however, this practice is not standard. As reported, greater hematological toxicities were observed in VEN + HMA which could have led to delays in completing the first or second cycle.
A number of patients with poor-risk cytogenetics were included in this study, and many demonstrated a response to therapy making up about 80% of patients who responded. Interestingly, a lower proportion of responding VEN + HMA patients had a complex karyotype compared to patients responding to HMA alone (59% vs. 70%). Previous data have shown that patients with TP53 mutations may particularly respond better to decitabine [17]. Consequently, these data have pushed common practice at our institution towards decitabine as the HMA of choice for poor-risk AML, and whether to add VEN remains debatable. It is likely that the choice of HMA could have influenced our results as patients who received HMA monotherapy were more likely to receive decitabine (92% vs. 8%), but in the VEN + HMA group, a higher number of patients received azacitidine (56% vs. 44%). Undoubtedly, larger studies that better represent patients with poor-risk disease are needed to capture the potential benefit, if any.
All five recipients of a subsequent bone marrow transplantation (BMT) were in the HMA group. Although only speculation, this may be explained by the decreased likelihood that patients who received VEN + HMA will sustain adequate count recovery to advance to transplant. Additionally, patients who remained aplastic or at most reached MLFS may not be good candidates for transplant overall. Finally, the increased toxicities seen with VEN + HMA could have precluded patients from receiving any additional therapy.
Periods of neutropenia and thrombocytopenia may have hindered subsequent dosing as a handful of patients never achieved count recovery. The prolonged cytopenias seen particularly in the VEN + HMA group can be difficult to elucidate as it could be a direct adverse effect of the therapy or it could be a sign of treatment failure and the presence or progression of disease. This relationship is most challenging during the first few cycles where many patients often have pancytopenia secondary to uncontrolled disease; however, the introduction of VEN + HMA could extend time to count recovery indefinitely in certain cases. Toxicities were greater with VEN + HMA with an overwhelming majority of patients who experienced grade 3/4 neutropenia. The rates of grade 3/4 neutropenia and thrombocytopenia in the total population were much higher in our study compared to the DiNardo reference study (100% vs. 71% and 94% vs. 53%, respectively). This led to frequent dose delays and reductions, but management strategies differed across practice - for some patients, VEN was reduced from 28 to 21 days per cycle whereas in other cases, the dose of VEN was reduced but the duration remained the same.
The use of granulocyte colony-stimulating factor (G-CSF) remains highly variable at our institution and has evolved over the course of the study period. This intervention was not assessed in our study; however, it would be interesting to compare hematological recovery in patients that received G-CSF versus those that did not and whether it resulted in fewer treatment delays and dose reductions. While the use of G-CSF may come with added controversy due to the theoretical risk of stimulating any remaining myeloid blasts in scenarios where morphological evidence of disease is unknown, this point of interest may be worth further exploring as a strategy to circumvent hematological toxicities and subsequent dose delays.
The rate of hospitalization for infection and/or febrile neutropenia was consistent with the high rates of neutropenia seen in both groups. The breakdown of infections consisted mostly of bacterial infections with a handful of suspected infections that were never confirmed and did not result in definitive therapy.
Most patients were started on appropriate antifungal prophylaxis with many following the recommended ramp-up schedule provided in the prescribing information for VEN. Seven patients received a VEN ramp-up that differed from the approved dosing schedule or did not receive a ramp-up at all, exclusively prior to FDA approval and the updated labelling. TLS occurred at a higher rate in our study compared to none reported by DiNardo et al. Of the three cases of TLS reported, one did require renal replacement therapy for acute renal failure that was attributed to VEN; however, the patient did not follow the ramp-up dosing schedule correctly, which was presumed to explain the severity of TLS. Another patient was assigned the correct dosing schedule, but due to TLS, was only ramped up to 100 mg. The third patient adhered to the assigned ramp-up of 200 mg with concomitant fluconazole.
The appropriate dose adjustment for VEN with concomitant antifungals has sparked great discussion. At our institution we recently changed the target dose for VEN from 70 to 100 mg when given with posaconazole out of concern for suboptimal response. Of note, this change occurred after data collection for this study was complete. As we accumulate more patients’ post-modification, it will be interesting to investigate if there are differences observed between patients that received 70 mg versus 100 mg of VEN concomitantly with posaconazole. How drug interactions, particularly antifungals, are addressed is something that continues to evolve and with more data becoming available with VEN in these populations, this is hopefully an area that will gain better clarity.
Limitations of this study were the retrospective nature, single-institution population, the small sample size and short follow-up period. There was variation among the dosing for VEN ultimately based on the timing of therapy in relation to FDA approval. As a result, the ramp-up schedule for VEN was not universally established initially, particularly in the presence of antifungals, which created differences in target doses achieved. The decision of which HMA to use was based on institutional and provider practice which could have introduced selection bias when evaluating criteria such as past HMA therapy and risk stratification. Finally, it is difficult to fully assess adherence with oral chemotherapy retrospectively.
With VEN + HMA use widely expanding, one unanswered question that remains is how it compares to intensive chemotherapy. Our institution frequently sees patients with high-risk disease or those who have multiple comorbidities where intensive chemotherapy and transplant consolidation is more controversial, making VEN + HMA an appropriate treatment option. Selection bias could make studying these two arms challenging as others have commented in their retrospective reviews [18], but prospective clinical trials are needed to determine if VEN + HMA could ever compete with the efficacy seen in cytarabine-based induction and consolidation.
The placement of VEN + HMA in therapy may continue to shift as new studies evaluate the combination of HMA with other targeted therapy. For example, with the recent FDA approval of ivosidenib in combination with azacitidine for first-line therapy, we may begin to see this treatment used upfront in patients with IDH1 mutations and reserve VEN + HMA for subsequent therapy. Montesinos et al evaluated the use of azacitidine and ivosidenib compared to azacitidine and placebo in 146 patients and reported an event-free survival of 22.9 vs. 4.1 months, respectively [19]. However, additional data are needed to directly compare these combination therapies against one another to shape best sequence.
Use of VEN in patients harboring targetable mutations may expand with more research as initial studies suggest VEN with azacitidine may still respond well in the presence of FLT3 or IDH mutations. While patients with targetable mutations did not make up a large percentage in our study, other studies have reported similar outcomes between newly diagnosed wildtype and mutated disease. For example, Konopleva et al reported a composite CR rate of 67% for both FLT3-mutant and FLT3 wildtype patients when comparing VEN and azacitidine to azacitidine alone, with FLT3-TKD patients obtaining higher CRc (CR + CRi) rates compared to FLT3-ITD (77% vs. 63%) [20]. Pollyea et al reported a similar pattern in improved response rates with the addition of VEN to azacitidine compared to azacitidine alone (63% vs. 31%) and suggested that patients with IDH1/2 mutations and poor risk cytogenetics appeared to still perform well compared to wildtype (intermediate-risk, 24.5 months; poor-risk NR vs. intermediate-risk, 19.2 months; 7.4 months) [21]. Furthermore, it will be valuable to explore triplet therapy in patients with targetable mutations as that has become a point of interest in practice. Recently, we have had a few select cases at our institution where VEN + HMA was used in combination with gilteritinib in relapsed FLT3-positive AML. Early phase studies have suggested safe and efficacious use with VEN + HMA alongside FLT3-targeted therapy; however, variation in drug selection, dose reductions and course length must be further deduced [22].
The efficacy findings in our study are largely consistent with what has been shown with use of this therapy. VEN in addition to HMA has already become the standard of care at our institution for older adults with AML who lack targetable mutations. However, the rates of neutropenia may disqualify many patients from remaining on this combination therapy. This study may prompt discussions and create opportunities for standardization of neutropenia management and dose adjustments with VEN. Given the toxicity concerns associated with VEN, greater selectivity regarding tolerability is to be expected when choosing appropriate candidates for this therapy, and increased monitoring is likely required. Future findings may collectively encourage revaluation of current package labelling and the proposed dose adjustments with concomitant antifungals.
Conclusion
The use of induction VEN and HMA may offer a clinical benefit in both lines of therapy when compared to HMA monotherapy, including those with adverse cytogenetics. However, VEN is associated with greater hematological toxicities, including prolonged and profound neutropenia. Further prospective trials are warranted to further evaluate its place in therapy and define best practices to manage toxicity.
Acknowledgments
None to declare.
Financial Disclosure
Use of REDCap for this study was funded by The Ohio State University Center for Clinical and Translational Science grant support (National Center for Advancing Translational Sciences, grant UL1TR002733).
Conflict of Interest
Julianna Roddy, PharmD, BCOP, is currently employed with Merck Research Labs, Merck & Inc. Her contribution to this study was completed while she was employed at the James Cancer Hospital, Ohio State University. James Blachly, MD, has performed consulting and/or advisory board work for AbbVie, AstraZeneca, Astellas, KITE Pharma, and INNATE Pharma.
Informed Consent
Not applicable.
Author Contributions
Tracelyn Freeman, Kiersten Williams, James Blachly and Julianna Roddy gathered primary data. Eric McLaughlin performed primary statistical analysis of the data. Tracelyn Freeman, Kiersten Williams, Marcin Puto, Allyson Waller, Eric McLaughlin, James Blachly and Julianna Roddy analyzed data and contributed writing to the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available with the article.
Abbreviations
AML: acute myeloid leukemia; HMA: hypomethylating agent; VEN: venetoclax; R/R: relapsed or refractory; CR: complete remission; ORR: overall response rate; CRi: complete response with incomplete count recovery; MLFS: morphologic leukemia-free state
| References | ▴Top |
- De Kouchkovsky I, Abdul-Hay M. 'Acute myeloid leukemia: a comprehensive review and 2016 update'. Blood Cancer J. 2016;6(7):e441.
doi pubmed - Ossenkoppele G, Lowenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125(5):767-774.
doi pubmed - Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556-561.
doi pubmed - Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, Hoppe G, et al. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012;53(1):110-117.
doi pubmed - Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, Claxton D, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017;18(8):1061-1075.
doi pubmed - Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, Stone RM, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
doi pubmed - DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, Swords R, et al. Durable remissions with Ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398.
doi pubmed - Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, Altman JK, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463-471.
doi pubmed - Venetoclax prescribing information. Genentech USA, Inc. Revised 6/2022.
- Wei AH, Strickland SA, Jr., Hou JZ, Fiedler W, Lin TL, Walter RB, Enjeti A, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277-1284.
doi pubmed - DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17.
doi pubmed - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - DiNardo CD, Maiti A, Rausch C, Pemmaraju N, Naqvi K, Daver NG, Kadia TM, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7:e724-e736.
doi pubmed - Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447.
doi pubmed - Common Terminology Criteria for Adverse Events v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- The Ohio State University Center for Clinical and Translational Science grant support (National Center for Advancing Translational Sciences, Grant UL1TR001070).
- Welch JS, Petti AA, Miller CA, Fronick CC, O'Laughlin M, Fulton RS, Wilson RK, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023-2036.
doi pubmed - Jamy O, Lin K, Worth S, Bachiashvili K, Rangaraju S, Vachhani P, Bhatia R. Hypomethylating agent/venetoclax versus intensive chemotherapy in adults with relapsed or refractory acute myeloid leukaemia. Br J Haematol. 2022;198(3):e35-e37.
doi pubmed - Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, Heuser M, et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N Engl J Med. 2022;386(16):1519-1531.
doi pubmed - Konopleva M, Thirman MJ, Pratz KW, Garcia JS, Recher C, Pullarkat V, Kantarjian HM, et al. Impact of FLT3 mutation on outcomes after venetoclax and azacitidine for patients with treatment-naive acute myeloid leukemia. Clin Cancer Res. 2022;28(13):2744-2752.
doi pubmed - Pollyea DA, DiNardo CD, Arellano ML, Pigneux A, Fiedler W, Konopleva M, Rizzieri DA, et al. Impact of venetoclax and azacitidine in treatment-naive patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761.
doi pubmed - Maiti A, DiNardo CD, Daver NG, Rausch CR, Ravandi F, Kadia TM, Pemmaraju N, et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J. 2021;11(2):25.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


